Discovery of how organs form explains fatal birth defects and informs cancer research

Symmetry in vertebrates only goes skin deep—many internal organs grow differently left to right. Cornell researchers have discovered a temporary molecular traffic system that starts embryo’s organs growing in the proper direction and without which devastating diseases and defects form. Their paper, featured on the cover of the September 30 issue of Developmental Cell, inspired an accompanying peer commentary and sheds light on the function of a little-known protein with a big role in organ formation.
The study describes the series of molecular signals that instruct the intestines to loop counterclockwise, ensuring that they can fit untangled into the abdomen. Emerging from research on the midgut in chicken embryos, the findings suggest how other vertebrates may form other asymmetric organs, including the heart, and reveal previously unknown behavior from a gene important in cancer research.
“What we’ve learned about how organs take shape reveals what may contribute to fatal birth defects and other diseases that arise when organs form at random, opening new paths for diagnosis and prevention,” said principal investigator Dr. Natasza Kurpios, developmental biologist at Cornell’s College of Veterinary Medicine. “It may also have broad implications for cancer research.”
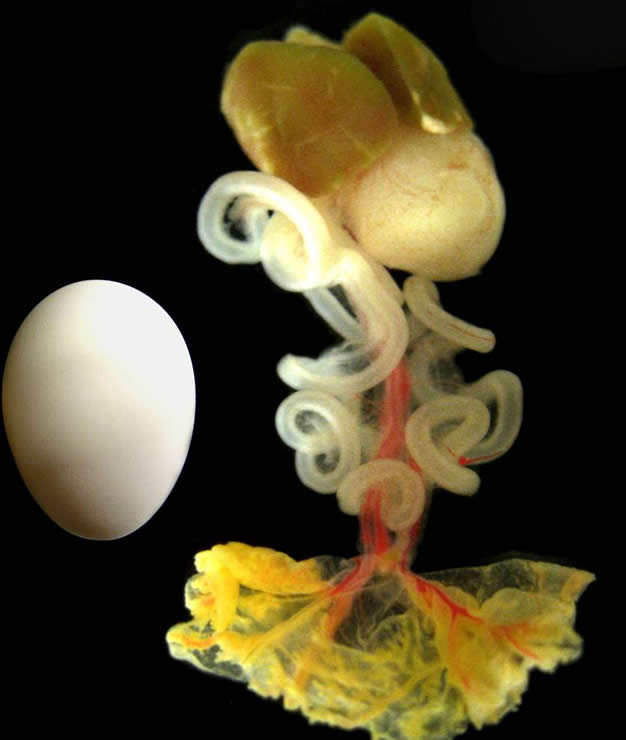
just prior to hatching (Day 18)
Embryos with randomly positioned organs do not survive. Called heterotaxia, this condition’s roots trace to mutations in the gene Pitx2, which is only found in the left side of the body. After determining how Pitx2 builds organs, Kurpios’ lab found that during a critical construction day early in intestinal growth, before the looping begins, Pitx2 directs production of a protein called Daam2 only on the left side of a harness-like tissue that holds the developing intestine in place.
The lock-like Daam2 then interacts with signaling by the key-like Wnt protein, arriving in a flood from the attached intestine. Together these players set up a temporary traffic-control system for intestinal cells. With Daam2 present only on the left, the effects of Wnt are felt only on this side. These effects are dramatic: In all species from humans to fruit flies, Wnt is crucial to cell proliferation, migration and multiple other cell behaviors, including tissue polarity.
“Wnt is critical for telling individual cells in an organ which way is up”, said Kurpios.
Ian Welsh, a graduate student in Kuprios’ lab and first author of the paper, found evidence that Daam2 was activated around the same time, suggesting a new role for Wnt in organ asymmetry. Once activated by Wnt, Daam2 directed and reorganized the growing number of intestinal cells to pack more tightly on the left side of the gut tube. This set the structure for the growing gut to start looping leftward.
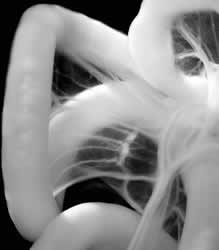
gut tube forms loops
These events occurred only for a day and entirely on the left side of the intestine. On the right side, Kurpios’ lab found inhibitors that disabled Wnt. The brief partnership between Wnt and Pitx2 occurred in gestation day 4 in chickens and day 10 in mice. They cooperated at exactly the right place at exactly the right time to start the intestines growing in the correct direction, and then never interacted again.
“This study will help clarify the molecular mechanisms of midgut malrotations [that] lead to devastating gut disorders,” said Drs. Olga Klezovitch and Valeri Vasioukhin of the Fred Hutchinson Cancer Research Center in the study’s accompanying commentary.
“Despite its broad importance, the ways Wnt controls cell behavior are still being worked out”, said Kurpios. “The discovery of Wnt’s partnership with Pitx2 and Daam2 may therefore also inform ongoing studies exploring Wnt’s role in a variety of cancers.”





