CVM scientists unveil a molecular dial controlling intestinal dynamics
The innermost lining of the intestine, referred to as the epithelium, is the most rapidly renewing tissue in the human body. Here one day, replaced five days later. How does it happen? The answer to this question has huge implications for human health: the gut is the site of nutrient absorption and the first line of defense against ingested pathogens.
In recent research, Dr. Praveen Sethupathy ’03 and colleagues identified a molecule that responds to changes in diet and regulates the growth of the intestinal epithelium. “The intestinal epithelial sheet is so critical to our health – what are the molecules and the pathways that control its proper growth and function?”
Consider how the gut environment changes throughout the day: every time a morsel of food is eaten, or a drink of water swallowed, the epithelial lining senses these changes and responds accordingly. The different types of cells within the epithelium are specialized to perform different functions, such as absorbing nutrients, maintaining energy balance, and defending against foodborne pathogens. There are even cells that interact with neurons in the gut and send signals to the brain that shape how we’re feeling. Stem cells in the epithelium give rise to various intermediate progenitor cell types, each of which then differentiate into specific specialized cell types. Because the epithelium replaces itself every three to five days, these stem cells are very active.
What controls stem cell activity remains an active area of research, says Sethupathy, and to address it, he turned to a specific class of
molecules known as microRNAs, which are important in controlling the rate of tissue growth in other organs, such as the pancreas, liver, and fat. They are also known to respond quickly to environmental changes, like diet. But little was known about the role of microRNAs in the intestine.
Under the guidance of Sethupathy, associate professor in the Department of Biomedical Sciences and director of the Center for Vertebrate Genomics, his team members Dr. Ajeet Singh, research assistant professor, Dr. Yu-Han (Amy) Hung, NYSTEM postdoctoral fellow, Dr. Mike Shanahan, research associate, and Dr. Matt Kanke, senior bioinformatics analyst, screened the epithelium for microRNAs using genomic tools. “Nobody had ever profiled microRNAs in all of the different cell types of the intestinal epithelium before, so we had a unique opportunity to paint the microRNA landscape of the gut,” Sethupathy said. Their studies picked up one particular microRNA, miR-7, as highly sensitive to dietary perturbations.
The team then ran experiments to study the function of miR-7 in mouse-derived enteroids, which are essentially “mini guts in a dish” that model the intestine outside the body. And, in close collaboration with Dr. Nicolas Buchon, associate professor in the Department of Entomology, and Dr. Allessandro Bonfini, a postdoctoral fellow in the Buchon lab, they also examined miR-7 within fruit flies. They found that both in mouse and flies miR-7 suppresses intestinal stem cell proliferation. The ongoing collaboration between the Buchon and Sethupathy labs has been highlighted at Cornell before.
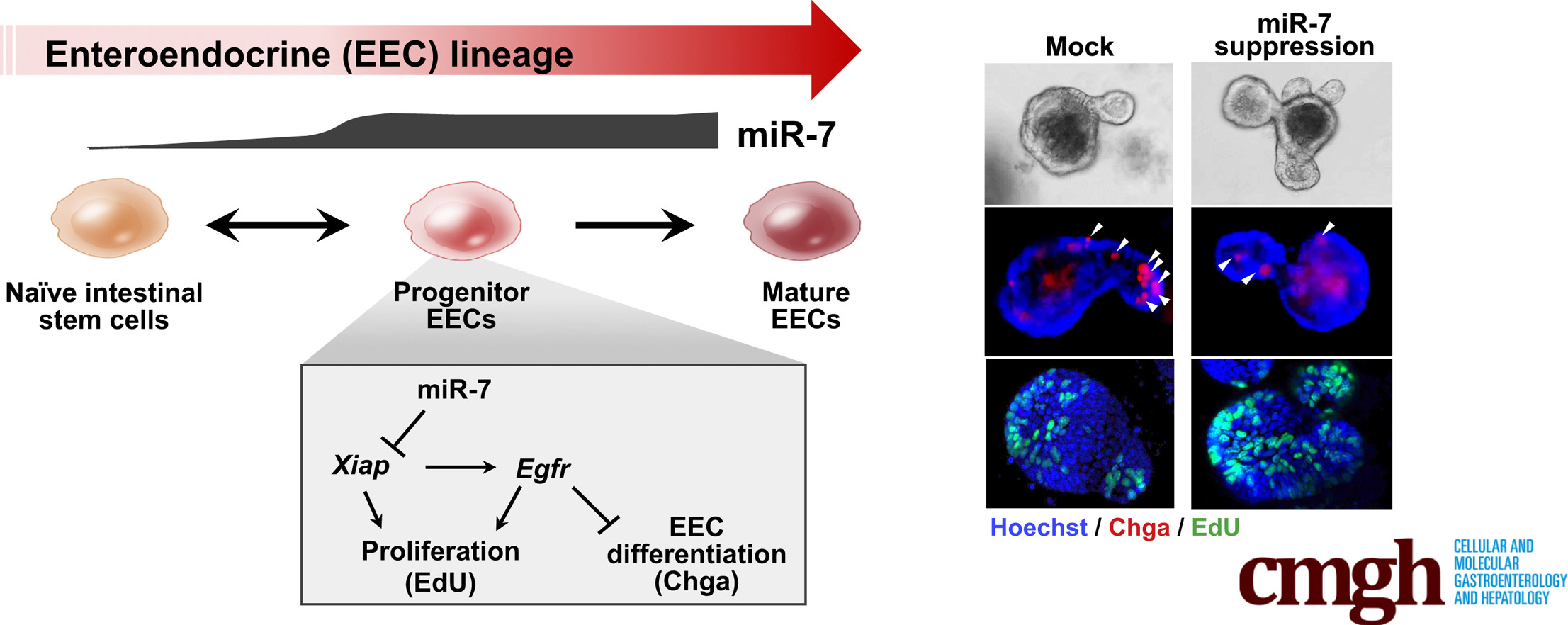
One of the key findings was that miR-7 is highly enriched in the progenitor cells that give rise to specialized cells with endocrine function, called enteroendocrine cells (EECs). “We were impressed with the level of specificity,” Sethupathy said. EECs comprise just one or two percent of the intestinal lining, but they have a vital, outsized role in maintaining energy balance. These cells sense gut contents, secrete proteins that regulate digestion as well as energy levels. Scientists hypothesize that these cells might be scarce or malfunctioning among people with type 2 diabetes.
“Enteroids are a powerful system for studying the function of specific molecules in the gut epithelium,” Sethupathy said. “Our results appear to suggest that by modulating the amount of miR-7, we could dial the production of EECs up and down,” Sethupathy said.
In other words, when miR-7 is abundant, there are fewer stem cells and more EECs. The opposite is also true: when miR-7 is limited, there are more stem cells and fewer EECs.
“This opens up several exciting research possibilities,” he said. For example, in bariatric surgery, which is a highly effective but invasive intervention for diabetes, there are nearly immediate, positive metabolic effects that are independent of weight loss, such as restored blood glucose levels. “One possibility is that after bariatric surgery, the number and the function of EECs increases thereby allowing for improved metabolic health. Could it be that miR-7 is part of the mechanism behind this?” he asked. “That’s one thing we’re excited about as a next step.”
Sethupathy has also generated a mouse that lacks two of the different versions of miR-7 that are encoded in the genome. “Now we’re in the midst of studying, in the live mouse, whether the effects are similar to what we observed in the enteroids. The results so far are very promising.”
The study, “Enteroendocrine Progenitor Cell–Enriched miR-7 Regulates Intestinal Epithelial Proliferation in an Xiap-Dependent Manner,” was published in the November 19, 2019 issue of Cellular and Molecular Gastroenterology and Hepatology.
-By Alison Fromme




