Humoral Immunodeficiencies
Impaired antibody production is the most frequently diagnosed immunodeficiency in mammals. In humoral immunodeficiencies, B cells either fail to develop or differentiate into antibody secreting plasma cells. In both cases, there is hypo- or agammaglobulinemia, and consequent susceptibility to infections with encapsulated bacteria.
Several humoral disorders have been described in the horse:
- failure of transfer of immunoglobulins at birth;
- transient hypogammaglobulinemia of the young;
- X-linked agammaglobulinemia;
- selective IgM deficiency;
- Fell Pony Syndrome;
- common variable immunodeficiency (CVID).
Failure in the transfer of immunoglobulins at birth
The failure in the transfer of immunoglobulins through colostrum results from maternal or neonatal factors, including pre-partum loss of colostrum, poor-quality colostrum, neonatal weakness, prematurity, immaturity, dysmaturity, perinatal hypoxia/ischemia, etc. Without immunoglobulins, the foal is at enormous risk to develop infectious diseases because of its immunologic naïve status.
Failure in the transfer of maternal immunoglobulins is characterized by serum IgG concentration less than 800 mg/dL in the foal 18 to 24 hours after ingestion of colostrum, and values between 400 and 800 mg/dL are considered partial failure. Serum IgG concentration can be quickly measured on the farm using SNAP-Test® (Idexx,Laboratories), which allows immediate decision for treatment with supplemental colostrum (if less than 18 hours of birth) or intravenous plasma transfusion.
Although the foal is capable of producing immunoglobulins as soon as it is exposed to environmental pathogens, it takes 5-8 weeks for endogenous immunoglobulins to achieve protective levels. Therefore, colostrum-derived antibodies at birth are essential for protection against pathogens. Partial transfer of immunoglobulins from colostrum combined with the active innate immune system of the foal may be protective for a short period of time, but soon the foal becomes susceptible to infections. The greater the transfer of colostrum immunoglobulins, more prolonged is the coverage with humoral protection until the foal produces its own protective levels of immunoglobulins.
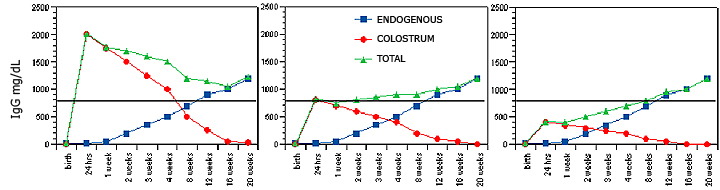
Hypothetical serum IgG concentrations in 24-hour old foals, including colostral IgG (red circles), foal endogenous IgG production (blue squares), and the sum of both (green triangles). The horizontal bar indicates minimal levels of protection (400-500 mg/dL). Figure 4A describes adequate transfer of immunoglobulins through the colostrum (starting at 2,000 mg/dL in the foal serum) and the physiological decay based on IgG half-life. Note that the colostral antibodies and the foal endogenous IgG production sum up to protective levels at the nadir between 8-12 weeks. In Figure 4B, despite lower (800 mg/dL) initial serum IgG concentrations in the newborn foal, the total IgG concentrations are still above protective levels, but the nadir is predicted to be 3-4 weeks. In Figure 4C, with initial serum IgG concentration of 400 mg/dL, the foal is already at risk in the first week of life. In all cases, a delay in endogenous antibody production in the foal creates a window of susceptibility, and the lower the initial colostral IgG concentration, the less time the foal has to compensate with endogenous production.
Transient IgG hypogammaglobulinemia of the foal
Foals are born hypogammaglobulinemic, with very low serum concentrations of IgM (< 30 mg/dL) and IgG (< 20 mg/dL), which do not confer protection against pathogens. Therefore, their immune system is naïve under the acquired immune point of view, and relies on the innate immunity and the passive transfer of immunoglobulins through colostrums at birth. A minimal post-suckle serum IgG concentration of 800 mg/dL is considered protective until endogenous antibody protective levels are reached by 5-8 weeks of life in healthy foals.
Transient hypogammaglobulinemia results from a delay in endogenous IgG production by the foal, which brings a risk of bacterial infection and poor development, particularly when colostrum-derived IgG concentrations decrease to levels below protection (< 500 mg/dL). Therefore, this condition is better diagnosed by 2-5 months of age, depending on the persistence of protective colostral immunoglobulin concentrations (colostral IgG half-life is 28-32 days). In general, this condition resolves by 5-6 months of life. Therefore, in foals with recurrent bacterial respiratory infections (Staphylococcus aureus, Streptococcus zooepidemicus, Klebsiella ssp, Actinobacillus equuli) from 3-5 months of life, the quantitation of serum immunoglobulin concentrations is advised to better understand their poor response to treatment. An increase in serum IgG concentration with time supports the transient condition, which is likely resultant from a delayed development of the immune system.
The cause of a delay in the development of the humoral immune response is unknown; perhaps it is associated with the lymphoid tissue milieu to support B cell differentiation. Peripheral blood B and T lymphocyte counts and distribution, and response to mitogen in vivo (skin) and in vitro are normal. Prolonged antibiotic therapy may be required during the hyporesponsive phase, and in severe disease, intravenous plasma transfusion.
X-linked agammaglobulinemia
Young male horses with this primary condition present agammaglobulinemia caused by impaired B cell development and, consequently, immunoglobulin production. Serum immunoglobulin isotypes (IgM, IgG, IgA) are often low or undetectable, depending on residual serum colostrum-derived immunoglobulin concentrations at the time of test (when < 6 months of life). Importantly, serum IgG, IgM and IgA concentrations do not increase over time. Clinically, the disease manifests around 4-5 months of life with recurrent fevers, bacterial respiratory and gastrointestinal infections. Most likely because T cell function is normal, the disease may be managed with antibiotic therapy for several months, until clinical complications result in death or euthanasia by the yearling age.
This disorder in horses presents many features of the X-linked agammaglobulinemia (XLA) described in male human patients and xid-mutant mice: a mutation of the btk gene on the X chromosome, which encodes the Bruton tyrosine kinase. The absence of this protein affects sustained signaling in response to B cell receptor engagement, leading to defects in B cell differentiation and proliferation. The outcome is the absence of circulating B cells, germinal centers and plasma cells in lymphoid tissues, and immunoglobulin production. Although there is no commercially available testing, the confirmation of a mutation in the btk gene may be done by gene sequencing.
Selective IgM deficiency
Selective IgM deficiency (with normal serum IgG and IgA concentrations) has been reported in horses of both genders and different breeds. It may manifest in the young (2 and 8 months of life) or adult horse. In any condition, serum IgM concentrations should be persistently and exclusively less than 25mg/dL, and IgG and IgA normal. If serum IgG concentrations are subnormal, then other forms of humoral disorders should be investigated. Transient conditions have been also diagnosed. Clinical signs are more severe in the young, and include recurrent fevers, pneumonia, arthritis, enteritis and generalized lymphadenopathy. In adult horses, lymphoma/lymphosarcoma, immunosuppressive therapy or stress may be present. The clinical implications of selective IgM deficiency in the adult horse are unclear because many horses do not show susceptibility to infections when their serum IgG concentrations is normal.
Peripheral blood B and T lymphocyte counts, and response to mitogens are normal. A genetic basis for this disorder has not been described. This condition is curious because immunoglobulin isotype switching to IgG and IgA must initiate from a mature B cell expressing IgM on the cell surface; therefore, the lack of IgM secretion, assuming the normal presence of IgM-expressing cells, is paradoxical.
Selective IgA deficiency
Even though selective IgA deficiency is recognized as an inherited genetic disorder with or without clinical susceptibility to infections in human patients, this disease has not been described in the horse in that form. Transient low serum IgA concentrations (with normal IgG and IgM) have been identified in horses but not persistently, i.e. serum concentrations return to normal values in subsequent tests. The susceptibility to infections during low serum IgA concentrations in horses is unknown; in addition, many human patients with this disorder are asymptomatic. Serum IgA concentration do not reflect necessarily mucosal IgA concentration, which is the site if its function.
Foal Immunodeficiency Syndrome
Foal Immunodeficiency Syndrome is a primary fatal immunodeficiency characterized by non-regenerative anemia, B cell lymphopenia, serum IgM hypogammaglobulinemia, and severe diarrhea, pancreatitis and bronchopneumonia in foals less than 2 months of life. The disease affects both genders of the Fell Pony and Dales breeds. Clinically, the foals may have no clinical signs at birth, with normal hematocrit and B cell distribution in peripheral blood. However, rapid non-regenerative anemia and sepsis develop, leading to death within weeks.
The immunodeficiency is associated with impaired antibody production due to a progressive B cell lymphopenia observed at primary and secondary (lymph nodes, spleen, mucosal-associate) lymphoid tissues. Serum IgG concentrations may be normal (> 800 mg/dL), reflecting circulating colostrum-derived antibodies at this age. However, serum IgM concentrations below 25 mg/dL support the diagnosis of impaired B cell differentiation and endogenous immunoglobulin production. Although the percentages of peripheral blood CD4+ and CD8+ T lymphocytes are normal, the in vivo T cell function is questionable in these foals, based on the absolute lymphopenia, poorly developed thymus and secondary lymphoid tissues, and infections with opportunistic organisms (e.g. Cryptosporidium parvum, adenovirus) despite normal circulating colostral IgG. The bone marrow reveals severe red cell hypoplasia, and abundant hemosiderophages, megakaryocytes and myeloid precursors. Peripheral ganglionopathy characterized by neuronal chromatolysis with nuclear pyknosis in the trigeminal, cranial mesenteric and dorsal root ganglia has been reported.
Pedigree analysis suggests inheritance through an autosomal recessive trait. The prevalence of carriers in the breed is estimated to be high. A genome-wide study identified a mutation in the gene SLC5A3 on chromosome ECA26 associated with the syndrome. The mechanistic implications of this mutation have not been fully resolved, and a causal relationship between the SLC5A3 mutation and FIS requires further studies to date. The presence of other mutations in that genomic region has not been ruled out as responsible for the phenotype of the immunodeficiency syndrome.The appropriate planning of breeding of carriers would prevent the outcome of affected foals and decrease the incidence of the putative mutant gene in the population.
Common variable immunodeficiency (CVID)
CVID is a humoral immunodeficiency described in the horse, characterized by late-onset B cell lymphopenia or depletion, hypo- or agammaglobulinemia, inappropriate response to tetanus toxoid vaccination, recurrent fevers and infections with encapsulated bacteria (pneumonia is the most common, followed by bacteremia, supurative meningitis, sinusitis, peritonitis, diarrhea, gengivitis, skin abscesses, and hepatitis). CVID has been diagnosed in unrelated horses between 2 and 23 years of life, with no sex, breed or blood line predilection.
In affected horses, serum IgM concentration is less than 25 mg/dL and does not change through time. Serum IgG concentrations are often below 800 mg/dL, and may oscillate between 200 and 800 mg/dL for months before levels become below detectable ranges. Serum IgA concentrations are often within normal range (between 75 to 120 mg/dL) despite low serum IgM and IgG concentrations, but progressively decrease with severity of disease. In vivo immunoglobulin production should be confirmed by pre- and 15 to 20 day post-tetanus toxoid vaccination serum IgG titers (see immunologic testing).
Lymphopenia (< 1,000 cells/uL) is a common presentation, and it may persistent or intermittent. Peripheral blood lymphocyte phenotyping reveals persistent B cell lymphopenia (< 2%), whereas CD4+ and CD8+ T cells are often normal to slightly increased. Occasionally, low CD4+ T cell distribution (45.9 ± 14.6% cells), with a low CD4/CD8 ratio (1.4 ± 0.4) accompanies the B cell lymphopenia. When present, CD4+ T cell lymphopenia seems to increase the susceptibility to fungal or intracellluar pathogen, with clinical disease (e.g. Pneumocystis carinii or Rhodococcus equi pneumonia).
The B cell lymphopenia seems progressive in these patients, to almost depletion from primary (bone marrow) and secondary (lymph node, spleen, mucosal-associated) lymphoid tissues. Therefore, the disease manifests clinically with susceptibility to infections in the adulthood. The disease is fatal, with some patients surviving for a few months or years on supportive therapy, once the diagnosis is made. Our laboratory has identified putative phases of B cell development that are affected in these horses, and seeks the gene defect that could explain and provide diagnosis of this disorder (see Research for further comments).


