Research
I concentrate on two different, but inter-related, research areas, which are the exploration into the mechanisms of thrombosis in animals and mechanisms of cancer metastasis in humans and animals. These two research areas are linked by a common theme of hemostasis, i.e. I am specifically looking at the role of coagulation in thrombosis and cancer metastasis.
Thrombosis in animals
Thrombosis is a serious complication of various diseases in animals, including sepsis, neoplasia, cardiac disease and inflammation. When thrombi develop in blood vessels, the tissues supplied by these vessels undergo hypoxic injury, causing cell death, organ dysfunction and even failure. The consequence of this tissue damage can be devastating to the patient, resulting in prolonged illness and even death. Thrombi are thought to form through three major pathways called Virchows's triad: endothelial injury, abnormalities in blood flow (stasis) and hypercoagulability. Hypercoagulability, which is defined as an abnormally activated coagulation system, is the research focus in my laboratory. I am particularly interested how tissue factor mediates hypercoagulability and, consequently, thrombosis in animals. Try searching for "tissue factor" on PubMed and see how many hits you get! You can also try alternate names for tissue factor, which are coagulation factor III (F3) and tissue thromboplastin - these really help narrow the search.
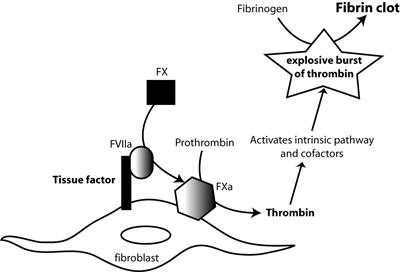
Tissue factor, in complex with factor FVIIa (FVIIa), activates factor X (FX). FXa, in the presence of calcium and factor V, cleaves prothrombin to thrombin. Thrombin amplifies its own production by activating coagulation factors (factor FIX) and cofactors (factor VIII, factor V) of the intrinsic and common pathways. This generates a large burst of thrombin, which then cleaves fibrinogen to fibrin and activates factor XIII, which crosslinks fibrin forming an insoluble fibrin clot.
Tissue factor is the main trigger of the complex coagulation cascade. It is a transmembrane protein that is constitutively expressed lining blood vessels. Coagulation is normally activated when endothelial cells are injured. This injury exposes extravascular tissue factor to its circulating ligand, plasma factor VII (FVII). Once bound to tissue factor, FVII becomes activated and together the two proteins form an enzymatic complex, which binds to and activates another coagulation factor, factor X (FX). FX is the lead protein of the common pathway of coagulation, whose end-result is the generation of the potent enzyme, thrombin, from prothrombin (factor II). Thrombin is the ultimate enzyme responsible for clot or thrombus formation, since it directly cleaves fibrinogen into fibrin, which forms the basis of thrombi (Figure 1).
With respect to thrombosis, I am studying the role of tissue factor and platelets in two main diseases - one affecting horses (I own a lovely Arabian gelding - see above - so I clearly have a personal agenda here) and one affecting dogs (really and truly man's best friend).
- Equine herpes virus type-1 (EHV-1) infection: Some horses infected with this virus develop a severe neurologic syndrome, called equine herpes virus myeloencephalopathy or EHM, which is characterized by paresis, paralysis and recumbency. Pregnant mares can also suffer from placentitis and abortion. A common pathologic finding in both of these disorders is the presence of thrombi in vessels within the spinal cord and placenta. My hypothesis is that the thrombosis is caused by an abnormally activated hemostatic system, with both platelets and tissue factor contributing to thrombus formation. In a study funded by the Morris Animal Foundation, we found that EHV-1 upregulates tissue factor on monocytes (see publication below). We have also made the novel discovery that EHV-1 activates platelets. Platelets are critical components in a thrombus, providing a large surface area on which thrombin formation proceeds (and is amplified). In a Grayson Jockey Club-funded proposal, we are currently testing various platelet inhibitors to see if we can stop EHV-1 from activating platelets. Our finding that EHV-1 activates platelets led to a new hypothesis, which we plan to test in the next few years with funding obtained through the Harold M Zweig Grants Program for Equine Research. This hypothesis is that platelets actually act as carriers of EHV-1 and can be sources of infection (a Trojan horse, if you will) for endothelial cells and monocytes.
- Immune-mediated hemolytic anemia (IMHA) in dogs. Dogs with this hematologic disorder develop severe anemia when their body starts to destroy its own red blood cells. During this disease, thrombi form in multiple blood vessels, such as the lungs. This thrombosis is a major cause of death in dogs with IMHA. Unfortunately, work in this area has slowed down, but I hope to get more funding to rejuvinate this important project.
Cancer metastasis
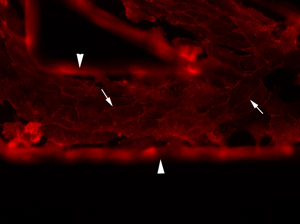
Endothelial cells grown within a fabricated microfluidic channel (arrowheads). The endothelial cells are confluent within the channel as shown by linear red immunostaining of vascular-endothelial cadherin (arrows), a marker of inter-endothelial junctions.
My interest in this area arose from my professional diagnostic service responsibilities as a clinical pathologist, in which I frequently make a diagnosis of cancer from aspirates of tissues of animals. My desire to understand the biology of cancer and to help animals led to this research focus in my laboratory. I am currently studying the role of tissue factor in cancer metastasis in humans and animals. Tissue factor is upregulated in cancer cells and is thought to be a marker of oncogenic transformation. Activation of coagulation, with thrombus formation, is a common consequence of cancer in people. We have shown that various different cancer cells from dogs express tissue factor. These studies have been extended by Lauren Witter and Erika Gruber (a clinical pathology resident). They have been working on thrombin generation by cancer cells and have data showing that these cells generate thrombin in plasma in a tissue factor-dependent manner (manuscripts in preparation). We have also been looking at how tissue factor helps tumor cells metastastize. To facilitate these studies, I established a collaboration with the Department of Biomedical Engineering at Cornell University. With the assistance of Dr. Michael Shuler, members of my laboratory (Drs. Mandy Esch and James Camp) have developed a unique microfluidic device, consisting of small (less than 70 um) semi-cylindrical "vascular" channels fabricated into a synthetic transparent polymer, called PDMS. Endothelial cells can be grown within the channels and can be exposed to fluid shear forces, similar to what they experience in blood (Figure 2). Sara Che, a graduate student in my laboratory, is using this device to explore how cancer cells interact proteins or endothelial cells to form metastatic tumors. Sara's funding is provided by an individual National Science and Engineering Research Center of Canada training grant and a National Institutes of Health Physical Science-Oncology Center (PSOC) program grant at Cornell University on the Microenvironment and Cancer Metastasis. Sara has found that high tissue factor-expressing cancer cells can bind to the natural inhibitor of tissue factor (called tissue factor pathway inhibitor) under static and fluidic conditions (using the above device), suggesting that this tissue factor-inhibitor binding could help capture circulating tumor cells to promote tumor metastasis, particularly in sites of low blood flow. Sara is now looking at how tissue factor-expressing microvesicles generated by cancer cells can affect endothelial cells to promote metastasis and she has had lots of help with this from great under-graduate students like Jeannie Park and Linda Wang.
Clinical pathology-related research
I am a veterinary clinical pathologist and am dedicated to advancing my field through investigative studies. Anything clinical pathology-related is fair research game, as can be seen from my varied research publications. My main clinical pathologic interests are hemostatic disorders and hematopoietic neoplasia.
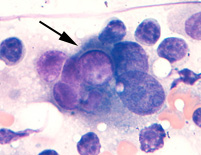
Panel A
Wright's-stained smear of a lymph node aspirate from a dog with a metastatic transitional cell carcinoma. A tumor cell with a purple intracytoplasmic inclusion is seen amongst lymphocytes.
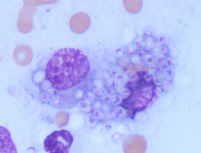
Panel B
Wright's stained smear of a skin lesion from a cat with Histoplasmosis. Many encapsulated yeast are seen within both macrophages in this image.
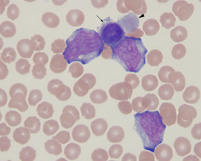
Panel C
Wright's stained blood from a dog with acute megakaryoblastic leukemia. There are three blasts along with a micromegakaryocyte (arrow) and giant bizarre platelet (arrowhead).


