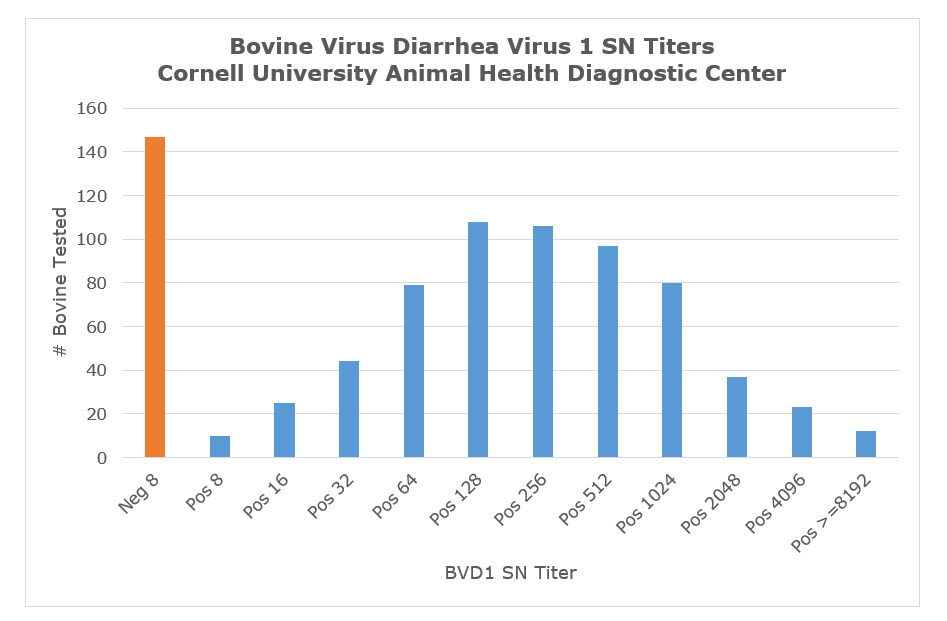
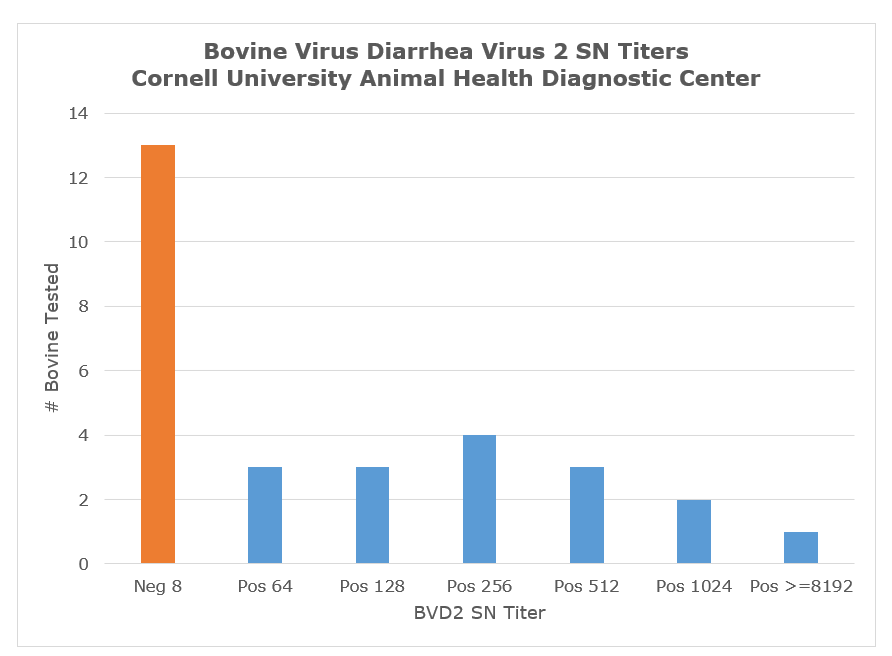
Bovine Viral Diarrhea Virus Serum Neutralization
Since the recognition of bovine viral diarrhea virus in 1946, the virus has undergone numerous classifications and name changes. Over the past 40 years, "bovine viral diarrhea virus" or BVDV became responsible for major economic losses to the bovine industry. Currently, BVDV is classified in the Flaviviridae family, Pestivirus genus which now has 11 species including BVDV (Pestivirus, A, B, H), border disease virus (Pestivirus D), and classical swine fever virus (Pestivirus C). BVDV type 1 (pestivirus A), BVDV type 2 (pestivirus B), and Hobi-like viruses (pestivirus H) cause major clinical respiratory and reproductive disorders in cattle worldwide.
Initial attempts to control BVDV by vaccination used several BVDV type 1 isolates such as Singer, NADL and C24V. Initial studies on serological cross-reactivity among BVDV isolates concluded that there were no defined serotypes and therefore all vaccines should be protective. The available vaccines did indeed prevent clinical disease in vaccinated animals challenged with field strains of the virus. With the identification of persistently infected (PI) animals that originate through vertical/in utero infection, "protection" of the fetus became a new criteria for evaluating the efficacy of vaccines. Shortly after the identification of the PI animal as a major factor in the spread of BVDV, a new BVDV virus referred to as BVDV type 2 was identified. Challenge of pregnant animals vaccinated with killed type 1 vaccines by BVDV type 2 clearly showed the need for inclusion of type 2 virus in vaccines. The cross-reactivity of antibodies against type 1 virus was not sufficient to prevent fetal infection by type 2 viruses. More recently a new species of BVDV was identified (Hobi-like viruses) in South America, Europe, and Southeast Asia. Limited studies strongly suggest that were this type of virus to be introduced into North America, currently available vaccines would be unlikely to confer fetal protection.
The interpretation of neutralization data related to BVDV is largely dependent upon knowledge of the vaccination history of the target animals. Most critical is the type of vaccine used – killed or modified live. Second major factor is the time post vaccination when the sample was collected. In general, killed vaccine titers may last several months post vaccination and range from 32-256 while modified-live vaccine titers can range from 512-1024. A non-immune animal infected with wild type virus will have titers early post infection in the low to mid-thousand range, but titers will decrease to titers similar to modified-live vaccinated animals. Animals vaccinated with killed vaccine and challenged with wild type virus will have early titers ranging from 5000-20,000 and will stabilize at values higher than modified live vaccinated animals. In general PI animals will have no or low Ab titers. As with all serological tests, testing of acute and convalescent serum samples may provide definitive proof of recent infection, based on anamnestic antibody titer increase between the acute and convalescent samples.
The AHDC offers routine testing for antibodies that neutralize BVDV types 1 & 2. Under special circumstances, we can screen for antibodies that recognize Hobi-like viruses. When deciding whether to test for antibodies for type 1 or 2 BVDV by SN, please consider the following:
- If the animal has been vaccinated with any strain of BVDV, there is no need to do both tests as an exposure will stimulate high cross-reacting antibody titers between BVDV-1 and -2.
- If there has been no vaccination (sentinel calf program), then both tests should be requested, as a low value to one type might show a 10-fold higher unequivocal titer to the other type. In this instance, the higher titer value would be suggestive of an infection with that type of virus.
The chart below represents neutralizing antibody titer values reported by the AHDC over the past 3 years. NOTE: Antibody titers at the AHDC are reported as the reciprocal of the dilution in which antibodies completely inhibited BVDV infection/replication (at 100% endpoint, e.g., 1:64 = Pos 64).