Canine Influenza Virus H3N2 Hemagglutination Inhibition
Canine influenza virus (CIV) is in the family Orthomyxoviridae, genus Alphainfluenzavirus, and included in the single species Influenza A virus. For taxonomic purposes, all influenza A viruses are considered equivalent regardless of the species of origin or HA or NA subtype. Currently there are 18 HA subtypes (1-18) and 11 NA subtypes (1-11). There is considerable confusion regarding the identification of influenza strains within the subtypes because the same HA and NA designations are used to identify viruses with different host ranges. For instance, there are avian H3N2 strains, human H3N2 strains, porcine H3N2 strains and canine H3N2 strains. While they share a core HA antigen relationship and genetic sequence relationship, they do not share a common host range, do not overlap in vaccine efficacy, nor share a common pathogenic potential.
Historically, the canine was not considered a significant host species of flu A viruses although there were sporadic reports suggesting human flu strains could infect dogs. This notion changed in 2004 with the isolation of an H3N8 virus from dogs with significant clinical disease. The origin of the virus was a direct link to the equine H3N8 lineage. Some significant epizootics occurred linked to the virus but over time the reports of H3N8 infections in dogs diminished significantly. In a similar timeframe, another canine influenza virus H3N2 emerged in Asia with reports from Korea to Thailand. This virus seemed to be more adapted to dogs as it grew to higher titers and spread more easily than H3N8. In the winter of 2015, the H3N2 virus was detected in the Chicago area and its genetic lineage indicated the virus originated in Korea. Since then there has been multiple introductions of this virus into the US by way of unethical transport of dogs from Asia.
The typical manner in which antibodies to influenza viruses are measured is the Hemagglutination inhibition (HI) assay. For this assay one must match closely the virus in the assay with the virus currently circulating. Even with the use of currently circulating virus, there may be differences in HI values related to the isolate of virus used in the assay. Antibodies generated against an H3N2 infection in dogs will cross-react with the H3N8 canine virus, but to a significantly lower value. For example, a serum with an HI titer to H3N2 of 1024 may present a titer of 32 against H3N8. Dogs vaccinated with the H3N8 vaccine and subsequently infected with H3N2 will show a substantial boost in the H3N8 titer along with a strong H3N2 titer. Interpretation of titer values must be done in the context of a vaccination history.
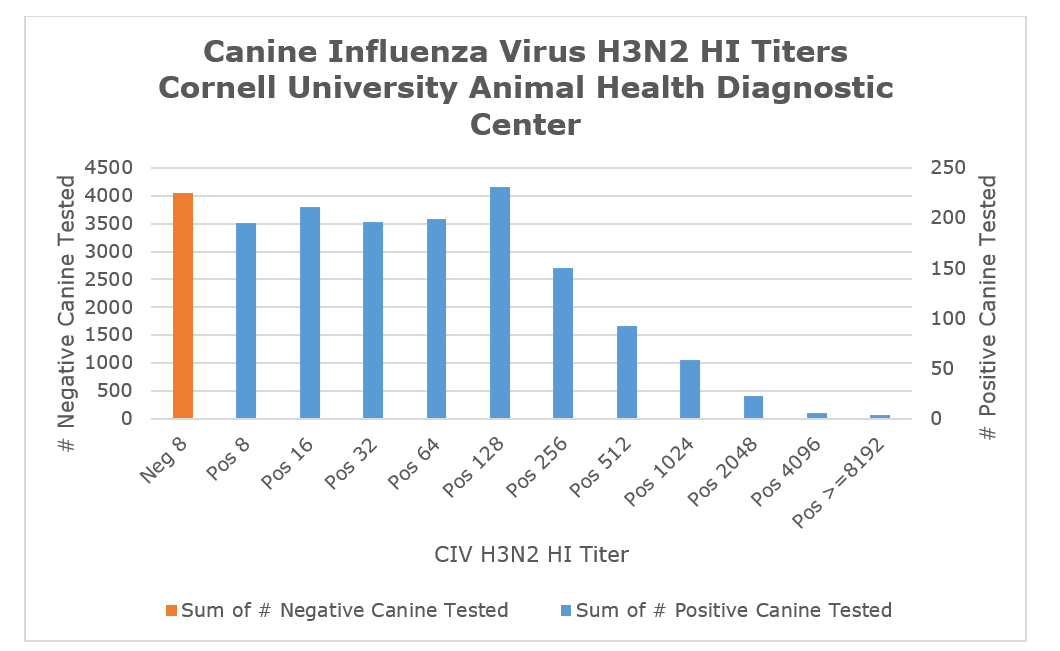
The chart below represents titer values for over 5400 sera tested for H3N2 since 2015. The seroprevalence among those samples was app. 25.3%, which is likely much higher than the overall canine population. As vaccines for H3N2 came into common use, the titer profile has shifted from values representing acutely infected animals to one representing mostly vaccine titers. Vaccine titers fall into the range of Pos 8 - Pos 128. Values over Pos 128 are likely to represent exposure titers. As with any serological test, titer values for acutely infected animals are impacted by the timing of the serum collection - early samples will show low values resembling vaccine titers whereas samples collected > 3 weeks post infection may present high antibody titers nearing the maximum levels of Pos 1024 - Pos 4096.