Canine Parvovirus-2 Hemagglutination Inhibition Test Data
Canine parvovirus virus-2 (CPV) or Carnivore protoparvovirus 1 is in the family Parvoviridae, subfamily Parvovirinae, genus Protoparvirus. Official taxonomic classification never did recognize that canine parvovirus, mink enteritis virus, or raccoon parvovirus were different from feline panleukopenia virus (FPV). The designation CPV-2 was necessary because a virus isolated earlier had the designation CPV-1 (minute virus of canines). CPV-1 is now classified as a canine bocaparvovirus. The origin of CPV-2 has engendered much speculation from being a mutant of FPV to a virus that may have come to the dog by way of raccoon parvovirus variants. Since its initial appearance in the dog, the host range of CPV-2 has expanded as a result of a series of genetic changes as it adapted to the canine host. Currently, the virus infects all members of the family Canidae (dogs, wolves, coyotes) as well as felines, mink, ferrets and raccoons. Genetic variants of the virus are known as canine parvovirus genotype 2a, 2b, and 2c, with the 2c genotype virus being the most recent to emerge. Vaccines currently in use are based on the 2a or 2b genotypes.
Canine parvovirus -2 is the most significant viral pathogen of dogs surpassing the mortality rates seen with canine distemper virus. In addition to its significant pathogenic potential, it has remarkable stability of infectivity in varied environmental conditions. Disinfection protocols that are effective for CPV-2 will handle virtually all other canine pathogens. The only truly effective way to prevent CPV-2 infections is to immunize susceptible dogs. Since the release of the first modified-live vaccine, it has been known that maternal antibodies transferred to puppies will interfere with the immunization process. Current vaccine protocols attempt to deal with this problem by recommending at least three vaccinations spaced 2-4 weeks apart. Even with this vaccine schedule, a few animals will fail to develop an effective immune response. There is no credible evidence that shows that currently circulating CPV-2 can cause clinical disease in properly immunized animals regardless of the vaccine used. Disease due to CPV-2 is a case of failure to immunize regardless of how many vaccines were given. It is important to keep in mind that vaccination is simply the physical process of delivering an antigen to an animal; immunization is the animal's response to the antigen exposure. Vaccination does not mean immunization in all instances.
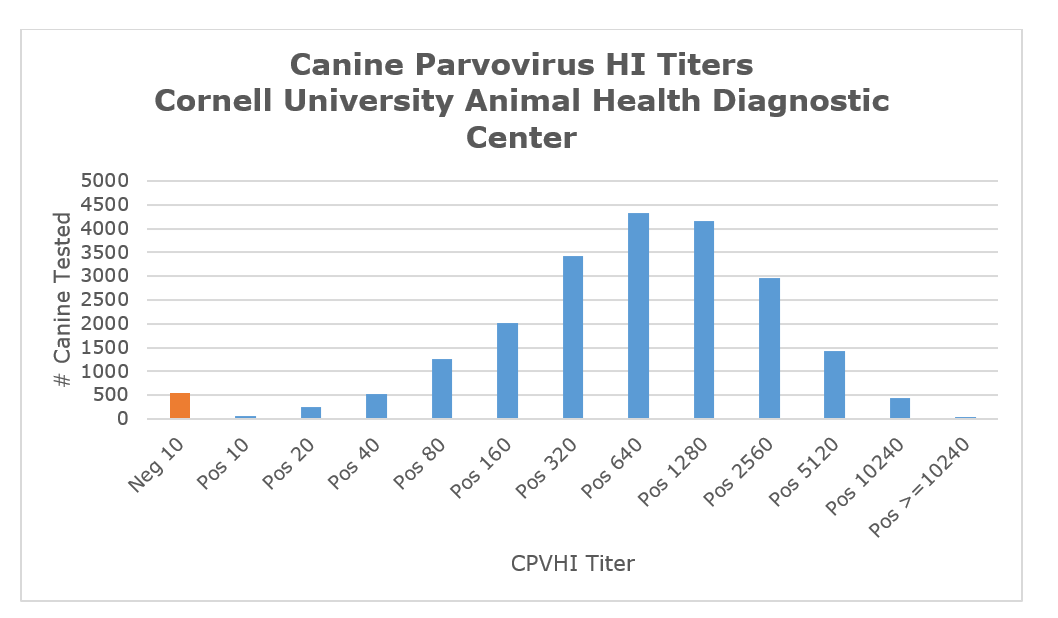
The chart below represents over 21,000 tests performed at AHDC over the past 3 years for CPV-2 by the Hemagglutination Inhibition (HI) assay. The vast majority of the tests were done on healthy animals to assess responses to vaccination. Many of the essentially negative animals (Neg 10) are from barrier raised animals for special studies. There are a few extremely high titers in dogs tested without clinical disease. The reason for this is unclear, but may be exposure to wild type virus early in the immune response to a vaccine.