Equine Influenza Virus H3N8 Hemagglutination Inhibition
Equine influenza virus (EIV) is in the family Orthomyxoviridae, genus Alphainfluenzavirus, and included in the single species Influenza A virus. For taxonomic purposes, all influenza A viruses are considered equivalent regardless of the species of origin or HA or NA subtype. Currently there are 18 HA subtypes (1-18) and 11 NA subtypes (1-11). There is considerable confusion regarding the identification of influenza strains within the subtypes because the same HA and NA designations are used to identify viruses with different host ranges. For instance, there are avian H3N8 strains and equine strains in several different clades. While they share a core HA antigen relationship and genetic sequence relationship, they do not share a common host range, do not overlap in vaccine efficacy, or share a common pathogenic potential.
Historically, there were two recognized equine influenza viruses. The first one described was an H7N7 (equine-1 or equine A-1) in 1957. Since about 1980, the virus seems to have disappeared from the equine population such that current vaccines no longer contain this subtype of EIV. In 1963, a second EIV was recognized and given the designation H3N8 (equine-2 or equine A-2). This subtype has continued to circulate in horses and has continued to evolve forming separate genetic clades. Currently in the US, the Florida sublineage clade 1 predominates while Florida sublineage clade 2 is found mainly in Europe, Africa, and Asia. With the evolution of the H3N8 viruses, efforts to monitor the antigenic shifts in the virus are ongoing in order to adjust the strains of virus in the vaccines.
The typical manner in which antibodies to influenza viruses are measured is the Hemagglutination inhibition (HI) assay. For this assay one must match closely the virus in the assay with the virus currently circulating. Even with the use of currently circulating virus, there may be differences in HI values related to the isolate of virus used in the assay. In the AHDC, we update the virus used in the HI assay to match a wild type virus recently isolated. As there are almost no reports of Florida clade 2 virus in the US, the testing at the AHDC uses exclusively Florida clade 1 virus.
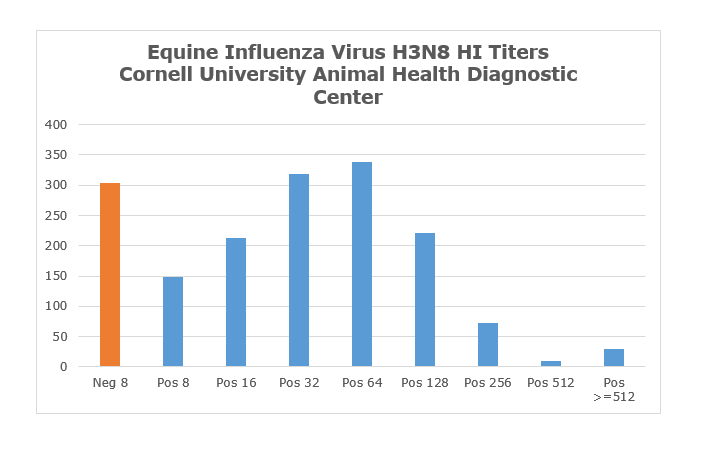
The chart below represents titer values for over 1600 sera tested for H3N8 since 2015. The seroprevalence among those samples was approximately 80%. As vaccines for EIV H3N8 are in common use, it is difficult to determine what proportion of these cases were from natural infections versus vaccination. For titers at 256 or above, one should keep EIV in the differential as these values are above normal vaccine titers. As with any serological test, titer values for acutely infected animals are impacted by the timing of the serum collection - early samples will show low values resembling vaccine titers whereas samples collected at 3-4 weeks post infection will be near maximum levels.