Canine Adenovirus 1 Serum Neutralization
Canine adenoviruses (CAdVs) are members of the Atadenoviridae family and Mastadenovirus genus. These viruses are classified as canine adenovirus type 1 (CAdV-1) and 2 (CAdV-2) based on distinct genetic and pathogenic characteristics in dogs. Canine adenovirus type 1 (CAdV-1) has a tropism for vascular endothelial cells and hepatocytes, replicating in the liver and causing acute hepatitis known as acute canine hepatitis. Canine adenovirus type 2 (CAdV-2) presents a tropism for epithelial cells in the respiratory tract causing infectious tracheobronchitis (ITB) and being part of the canine infectious respiratory disease (CIRD) complex. In addition to domestic dogs, CAdV-1 has been detected in several species of wild animals including foxes, wolves, racoons, river otters, coyotes and brown bears, while CAdV-2 has been detected in brown bears, racoons, dogs, cats and wolves.
Notably, despite major differences in pathogenesis, CAV-1 and CAV-2 are serologically related, and antibodies elicited in response to infection or vaccination with one virus cross react and cross neutralize the other. Indeed, the canine adenovirus vaccines currently available for use in dogs in the US are based on a low virulence CAdV-2 strain. Its use, however, is primarily indicated for prevention of CAdV-1 (against which it cross-protects), which causes severe hepatitis usually leading to death in naïve dogs.
Serological testing for CAdVs can be used to detect exposure to the virus, diagnosis of acute infection (if paired samples collected 2-3 weeks apart are tested), to check for antibody responses following immunization or to help veterinarians to determine if a booster immunization is warranted. Diagnosis of acute infection, in the absence of a vaccination history, can be reached if a fourfold rise in antibody titer over a 2- to 3-week period is observed together with compatible clinical signs. Although protective neutralizing antibody levels have not been definitively determined, experimental studies have shown a correlation between high neutralizing antibody titers (>500) and protection from acute hepatitis induced by CAdV-1 in dogs. It is important to note that the presence of CAdV neutralizing antibodies pre-vaccination or pre-booster may prevent titer increases after vaccination, as the pre-vaccination antibodies will reduce or prevent vaccine take.
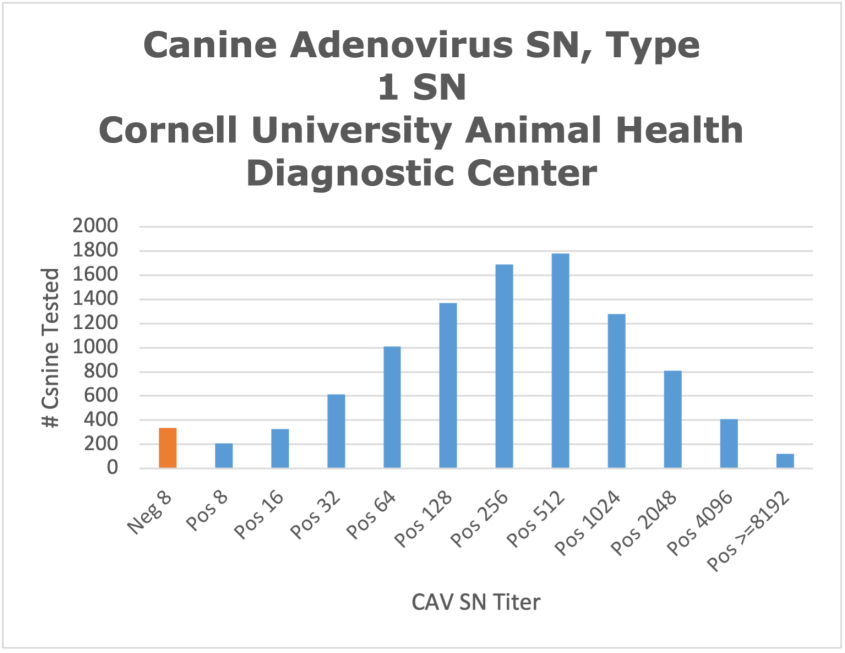
The chart below presents CAdV-1 neutralizing antibody titers of over 9,900 SN tests performed in canine serum samples over a 5-year period (2020-2025) at the AHDC. Most of these submissions represent healthy animals for which vaccine or maternal titers were being assessed. The AHDC uses a strain of CAdV-1 on the SN test, since this is the virus associated with severe clinical manifestations in dogs and other carnivores. Antibody titers at the AHDC are reported as the reciprocal of the highest serum dilution that completely inhibits CaDV-1 infection/replication (at the 100% endpoint, e.g., 1:64 = Pos 64). Antibody titers can be interpreted as Low: Neg 8 to Pos 32; Intermediate Pos 64 to Pos 256; or High for titers ≥512.