Total Protein Electrophoresis
This procedure separates the proteins in serum and body fluids (e.g. peritoneal fluid, urine) into the component albumin and globulins. Electrophoresis is indicated for determination of the underlying nature of a hyperglobulinemia or if multiple myeloma is suspected in a patient. The types of immunoglobulin comprising a hyperglobulinemia can provide useful diagnostic information. It is less useful for evaluation of immunodeficiencies; individual immunoglobulin quantification provides more information in these disorders. Currently at Cornell University, we are using capillary electrophoresis for our protein electrophoresis. This is a very sensitive technique and splits the proteins into multiple fractions, which are species-dependent. For simplicity of reporting, we do not report out results for individual fractions, but those for combined fractions, e.g. albumin, a1, a2, total a, b1, b2, total b and g. We provide interpretative comments on our electrophoretograms as well.
Electrophoresis Components
The electrophoretogram splits the protein fraction of serum or plasma into its constituent components this includes albumin and globulins. There are three globulin fractions: alpha, beta and gamma.
Total protein
This is measured on the Cobas C501 using the biuret method (serum, plasma, body fluids) or turbidometry (CSF, urine). A total protein concentration is necessary to provide absolute values (not just percentages) of the protein components.
Albumin
Albumin is the first peak on the electrophoretogram and is usually a tall thin peak. The albumin concentration by ELP is usually lower than that from the Modular P.
Alpha (a) globulins
These migrate next to albumin and are synthesized in the liver. They include the acute phase reactant proteins, a-2 macroglobulin and haptoglobulin. In most species, a-globulins can be split into two main components, a1 and a2, although further subdivisions are present in some species or individual animals.
Beta (b) globulins
These migrate between g- and a-globulins. They are usually produced in the liver and include fibrinogen (plasma), transferrin and complement components. Like a-globulins, b-globulins can be divided into two major components (b1 and b2) in most species, although further subdivisions are evident (e.g. dogs usually have b1a and b1b).
Gamma (g) globulins
This comprises the immunoglobulins, IgG, IgA and IgM and is the furthest peak from albumin. In reality, IgA and IgM often migrate in the late b- (b2-region) or early g-region. The shape of the g-peak provides diagnostic information. A broad-based peak indicates a polyclonal gammopathy (see panel B below), which is usually due to antigenic stimulation and is not disease specific. A tall sharp peak in the g-region or late b-region is compatible with a monoclonal gammopathy. Monoclonal peaks are usually due to neoplastic disorders, e.g. multiple myeloma, B cell lymphoma, or B cell chronic lymphocytic leukemia. In rare cases, inflammatory or infectious diseases can present with a narrow gamma peak which can be difficult to distinguish from a true neoplastic monoclonal gammopathy. This is more correctly termed a "restricted oligoclonal" gammopathy, and has been reported in various diseases such as Ehrlichia, Leishmania, FIV, FIP, and other diseases.
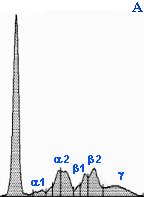
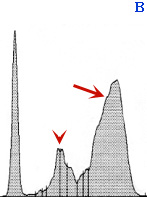
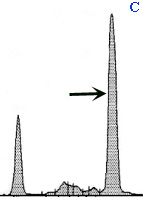
Different electrophoretogram patterns in serum
Panel A: Normal agarose gel electrophoretogram in a dog. The tallest peak to the left is albumin, followed by a1 (2 peaks), a2 (2 peaks), b1 (2 peaks of b1a and b1b), b2 and g(the last flat peak).
Panel B: Serum from a cat with feline infectious peritonitis virus (FIPV) infection. There is an increase in a-2 globulins (arrowhead), indicating an acute phase reactant response, and a polyclonal gammopathy (arrow). These results are typical, but not specific, for FIPV infection (they can be seen with other inflammatory conditions).
Panel C: Serum from a dog with multiple myeloma. There is a tall narrow peak in the g-region, indicating a monoclonal gammopathy (arrow). Albumin concentrations are also decreased (compare to the normal dog in panel A).
Serum protein electrophoresis
Serum is the preferred sample for electrophoresis. Fibrinogen (in heparinized samples) produces a monoclonal peak in the b-region, which affects interpretation in plasma samples. We have established our own reference intervals for dogs, cats, horses, cattle and alpacas. Therefore, abnormal results in these species will be flagged. In addition, we always provide the electrophoretogram scan itself, not just the written report.
Body fluid protein electrophoresis
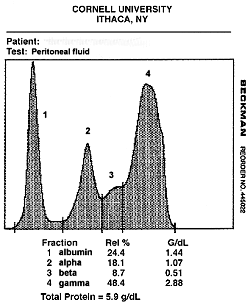 Electrophoresis can be performed on body fluids, such as peritoneal fluid. This is usually done in cats with abdominal effusions to support the diagnosis of feline infectious peritonitis virus infection. In this disorder, an exudative effusion can develop in the abdomen. The electrophoretic characteristics of the fluid is similar to the serum results as the effusion is due to a vasculitis. Therefore, serum and fluid should be submitted concurrently for electrophoresis. Both usually show an increase in a-globulins (acute phase response) and a polyclonal gammopathy (broad-based increase in g-globulins). An increase in g-globulins in the fluid is suggestive for FIPV infection (see image below). Electrophoresis can also be done on CSF samples, however this is not done routinely because large volumes of CSF are required. The protein in CSF is usually too low to run on ELP without concentration. We need a minimum of 600 mg/dL of protein to perform electrophoresis. Concentrating CSF requires at least 5 to 10 ml of fluid, which cannot be obtained from many animals.
Electrophoresis can be performed on body fluids, such as peritoneal fluid. This is usually done in cats with abdominal effusions to support the diagnosis of feline infectious peritonitis virus infection. In this disorder, an exudative effusion can develop in the abdomen. The electrophoretic characteristics of the fluid is similar to the serum results as the effusion is due to a vasculitis. Therefore, serum and fluid should be submitted concurrently for electrophoresis. Both usually show an increase in a-globulins (acute phase response) and a polyclonal gammopathy (broad-based increase in g-globulins). An increase in g-globulins in the fluid is suggestive for FIPV infection (see image below). Electrophoresis can also be done on CSF samples, however this is not done routinely because large volumes of CSF are required. The protein in CSF is usually too low to run on ELP without concentration. We need a minimum of 600 mg/dL of protein to perform electrophoresis. Concentrating CSF requires at least 5 to 10 ml of fluid, which cannot be obtained from many animals.


