Parasitology
For more information on tests performed by the Parasitology section, please refer to the information below.
Tests
Baermann Technique
| Category | Description |
|---|---|
| Test Code: | BAERF |
| Test Name: | Baermann Fecal Technique |
| Test Method: | Nematode larval identification |
| Sample Required: | 10 grams of feces |
| Collection container: | plastic leak proof container |
| Transport: | Ship on cold packs |
| Test Day: | M-F |
| Lag Time: | 2-3 days |
| Species: | All species |
| Results Format: | Positive with identification of nematode larvae detected, or Negative for parasites |
Interpretation
This technique is a modification of the Berlese Apparatus used by entomologists to collect insects from plant material and soil. It is used to retrieve nematode larvae from feces, soil, plant matter or other organic material. The Baermann Technique operates on the principle that the nematode larvae wiggle out of the biological material, cannot swim against gravity and will fall through the water to the area of clamped off tubing. The clamp is released to collect the larvae for identification. Any fecal sample submitted for this procedure must be freshly voided so that the sample is not contaminated by free-living nematodes.
The Baermann Technique is not recommended as a primary diagnostic technique for evaluation of parasites in feces. It is not useful for those nematode larvae that do not leave the feces or other biological material, or for detection of parasite eggs or cysts that may be in the fecal sample. Some lungworm larvae are retrieved using this technique, but some larvae such as those of Filaroides hirthi, Filaroides osleri, Strongyloides sp.and Dictyocaulus sp. are better recovered using flotation techniques. Lungworms, such as Eucoleus (Capillaria) aerophilus, that do not produce larvae are detected only by using flotation methods as well.
Cryptosporidium ELISA Test
| Category | Description |
|---|---|
| Test Code: | CRYPTO |
| Test Name: | Cryptosporidium ELISA |
| Test Method: | Antigen capture enzyme-linked immunosorbent assay |
| Sample Required: | 10 grams of feces |
| Collection container: | plastic leak proof container |
| Transport: | Ship on cold packs |
| Test Day: | Wednesday |
| Lag Time: | 1-8 days |
| Species: | All species |
| Results Format: | Positive or Negative |
Interpretation
The Alexon Cryptosporidium Monoclonal Antigen Capture Enzyme-linked Immunosorbent Assay (ELISA) is a fecal procedure designed to detect a Cryptosporidium specific antigen. This procedure is done in parallel with a double centrifugation concentration flotation procedure for Cryptosporidium oocysts and any other parasites that may be present in the fecal sample.
The results of this test can be interpreted as follows:
- Cryptosporidium ELISA Positive/Flotation Positive
The animal is infected with Cryptosporidium. - Cryptosporidium ELISA Positive/ Flotation Negative
The animal may be infected with Cryptosporidium but is shedding oocysts below the limits of detection by flotation. Alternatively, the ELISA is a false positive that may be seen most frequently when the results are in the low positive range. To resolve the issue, collect a second sample for analysis. - Cryptosporidium ELISA Negative/ Flotation Positive
The animal may be infected with Cryptosporidium but is producing antigen below the limits of detection by ELISA. Alternatively, the ELISA is a false negative. To resolve the issue, collect a second sample for analysis. - Cryptosporidium ELISA Negative/ Flotation Negative
The animal is not infected with Cryptosporidium.
Fecal Egg Count Reduction
| Category | Description |
|---|---|
| Test Code: | FECRT |
| Test Name: | Fecal Egg Count Reduction |
Request a fecal floatation test (Test code: FLOAT) for day-0 fecal samples from individual animals (usually heavy shedders). A discount is available when submitting six or more fecal samples in one submission (Test code: FLOAT6). Use the general AHDC submission form for day-0 samples.
Request a fecal egg count reduction test (Test code: FECRT) for the follow-up post-treatment sample (usually day 14). Follow the link http://bit.ly/FecalEggCount in the original FLOAT report to access submission form for FECRT. Note that FECRT submission form must be completed to receive the discounted price.
Fecal Egg Count Reduction Test (FECRT) based management of anthelmintic resistance in equine herd.
FECRT is the current gold standard test to detect and monitor emergence of resistance to commonly used drugs against gastrointestinal helminth (strongyle) parasites. The test compares strongyle egg count in feces before- and 10-14 days after- anthelmintic treatment and is expressed as percent egg reduction.
Anthelmintic resistance has emerged as serious threat to livestock worldwide, especially to horses. To date, resistance has been reported for all class of drugs used for equine strongyle infection. Monitoring resistance in a herd would help design appropriate treatment strategies aimed to mitigate the impact of resistance in a herd.
Before embarking on treatment strategy based on FECRT, one must first determine the egg shedding potential of each horse in a herd. Not all horses are equally infected. In fact, 50-75% of horses in any herd are low egg shedders as noted in the chart below. Horses are genetically destined to shed strongyle egg, meaning a low shedder typically remains a low shedder throughout its life.
| Classification based on egg shedding | Egg count (per gram of feces) | % of horse population |
|---|---|---|
| Low | 0-200 | 50-75 |
| Moderate | 200-500 | 10-20 |
| Heavy | >500 | 15-30 |
The egg shedding potential of a horse is determined by performing fecal egg count (FEC) at least two times in a year: once during early spring and another at fall. Designate each horse as low, moderate or heavy shedders as per the classification chart above. It is important that sampling of horse must be done prior to any drug treatment or if recently treated must wait until the egg reappearance period (ERP). ERP indicates new infection from pasture as the efficacy of recent treatment fades out. Refer chart below for equine strongyle ERP for common anthelmintic drugs.
| Anthelmintic | Usual ERP when drug is effective (weeks) | ERP when drug was first introduced |
|---|---|---|
| Fenbendazole | 4-5 | 6 |
| Pyrantel | 4-5 | 5-6 |
| Ivermectin | 6-8 | 9-13 |
| Moxidectin | 10-12 | 16-22 |
Once the shedding potential of horses in a herd is determined, treat only the heavy shedders (FEC>500 eggs per gram of feces). The low shedders are left untreated while the moderate shedder is kept on watch. Untreated low shedders continue to maintain a ‘refugia’ of susceptible worm population in a herd that potential dilute the number of resistant larvae in the pasture.
FECRT is performed every time when an animal is subjected to anthelmintic treatment. Perform a Fecal Egg count (EPG) prior treatment (day-0) and repeat FEC 10-14 days after treatment. Calculate the percent egg reduction post treatment using the formula below.
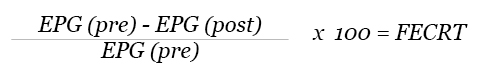
Refer the chart below to determine the efficacy of anthelmintic against equine strongyles:
| Anthelmintic | Expected efficacy if no resistance | Observed results of FECRT | ||
|---|---|---|---|---|
| N/A | Susceptible (no evidence of resistance) | Suspected resistant | Resistant | |
| Benzimidazole | 99% | >95% | 90-95% | <90% |
| Pyrantel | 94-99% | >90% | 85-90% | <85% |
| Ivermectin/Moxidectin | 99.9% | >98% | 95-98% | <95% |
Although AAEP recommends treatment of heavy shedders, it is advisable to do a whole herd treatment once in a year to tackle emergence of large strongyles and to control other parasitic infections such as bots.
Qualitative Fecal Flotation
| Category | Description |
|---|---|
| Test Code: | FECQL |
| Test Name: | Fecal Qualitative |
| Test Method: | Double centrifugation concentration fecal flotation |
| Sample Required: | 10 grams of feces |
| Collection container: | plastic leak proof container |
| Transport: | Ship on cold packs |
| Test Day: | M-F |
| Lag Time: | 1-3 days |
| Species: | All species |
| Results Format: | Identification of parasites detected in feces, or No Parasites Detected |
Interpretation
The qualitative fecal flotation, using a double centrifugation concentration technique, is a general broad based test for evaluating feces for parasitic infections. It indicates the presence or absence of patent protozoan or worm infections in domestic and wild animals of all species. Samples from individual animals are preferred. Composite samples tell when there is a parasite problem, but not the animals that are particularly affected.
In this laboratory, all samples are actively floated (centrifuged) using a sugar solution (1.33 specific gravity). Zinc sulfate solution (1.18 specific gravity) is used also for samples from small animals, animals under six months of age, and when delicate protozoa, such as Giardia, or worms, such as nematode larvae, are of concern. Using these flotation media, protozoan cysts and worm eggs and larvae are recovered for microscopic evaluation.
When submitting a sample provide a sufficient sample to make a diagnosis possible, and include a history of the health concerns related to the animal(s) from which the sample(s) are collected. For best results, collect fresh samples, keep them refrigerated, and submit them for examination within seven days of collection. Samples that are preserved in 10% formalin or 70% alcohol can also be submitted, but the preservative may compromise the quality of the sample for parasite detection.
Quantitative Fecal Flotation
| Category | Description |
|---|---|
| Test Code: | FECQN |
| Test Name: | Fecal Quantitative |
| Test Method: | Double centrifugation concentration fecal flotation |
| Sample Required: | 10 grams of feces |
| Collection container: | plastic leak proof container |
| Transport: | Ship on cold packs |
| Test Day: | M-F |
| Lag Time: | 1-3 days |
| Species: | All species |
| Results Format: | Identification of parasites detected with the number per gram of feces, or No Parasites Detected |
Interpretation
The quantitative fecal flotation, a double centrifugation concentration technique, is used to estimate the number of worm eggs or larvae, and protozoan cysts per gram of feces in a sample. Samples from individual animals are preferred. The results of this test can help determine whether a treatment is effective, the shedding status, or if drug resistance is developing. Composite samples tell when there is a parasite problem, but not the animals that are particularly affected, the intensity of the parasite burden in an animal, or the amount of environmental contamination that may be occurring. A quantified fecal examination is used primarily for large animals such as horses, cattle, sheep, goats and camelids, but fecal samples from all species can be quantified, if requested.
In this laboratory, all samples are actively floated (centrifuged) using a sugar solution (1.33 specific gravity). Zinc sulfate solution (1.18 specific gravity) is used also for samples from animals under six months of age, and when delicate protozoa, such as Giardia, or worms, such as nematode larvae, are of concern. Using these flotation media, protozoan cysts and worm eggs and larvae are recovered for microscopic evaluation. If the sample is diarrheic, it cannot be quantified, so a qualitative fecal flotation will be done instead.
When submitting a sample provide a sufficient sample to make a diagnosis possible, and include a history of the health concerns related to the animal(s) from which the sample(s) are collected. For best results, collect samples recently voided from the animal(s), keep them refrigerated, and submit them for examination within seven days of collection.
A Nematode Larval Culture may also be requested to identify to genus and/or species level any “strongyle” eggs detected in the sample. If you request a Nematode Larval Culture send a larger (15-20 gram) sample. Do not refrigerate the sample if you request a Nematode Larval Culture.
Giardia ELISA Test
| Category | Description |
|---|---|
| Test Code: | GIARD |
| Test Name: | Giardia ELISA |
| Test Method: | Antigen capture enzyme-linked immunosorbent assay |
| Sample Required: | 10 grams of feces |
| Collection container: | plastic leak proof container |
| Transport: | Ship on cold packs |
| Test Day: | Wednesday |
| Lag Time: | 1-8 days |
| Species: | All species |
| Results Format: | Positive or Negative |
Interpretation
Alexon Giardia Monoclonal Antigen Capture Enzyme-linked Immunosorbent Assay (ELISA) is a fecal procedure designed to detect a Giardia specific antigen. This procedure is done in parallel with a double centrifugation concentration flotation procedure for Giardia cysts and any other parasites that may be present in the fecal sample.
Giardia ELISA Positive/Flotation Positive
The animal is infected with Giardia.
Giardia ELISA Positive/Flotation Negative
The animal may be infected with Giardia but is shedding cysts below the limits of detection by flotation. Alternatively, the ELISA is a false positive that may be seen most frequently when the results are in the low positive range. To resolve the issue, collect a second sample for analysis.
Giardia ELISA Negative/Flotation Positive
The animal may be infected with Giardia but is producing antigen below the limits of detection by ELISA. Alternatively, the ELISA is a false negative. To resolve the issue, collect a second sample for analysis.
GiardiaELISA Negative/Flotation Negative
The animal is not infected with Giardia.
Modified Knott's Heartworm Technique
| Category | Description |
|---|---|
| Test Code: | KNOTT |
| Test Name: | Knott’s Heartworm Test |
| Test Method: | Microscopic identification |
| Sample Required: | 1 ml of whole blood in anticoagulant, such as EDTA or heparin |
| Collection container: | blood tube |
| Transport: | Ship on cold packs |
| Test Day: | M-F |
| Lag Time: | Results same day |
| Species: | All species that may have microfilariae in their blood |
| Results Format: | Identification of microfilariae, or No microfilariae detected |
Interpretation
This technique is used for the detection and identification of blood-borne microfilariae. It was developed for the detection of Dirofilaria immitis microfilariae in canine blood. In this country the microfilariae of Dirofilaria and those of Dipetalonema reconditum must be differentiated. Dirofilaria immitis microfilariae can persist in a positive animal that has been treated for up to six months after treatment.
The Knott’s Technique is not recommended as a stand alone diagnostic test for Dirofilaria immitis because infections may consist of male worms that do not produce microfilariae, or immature female worms that are not yet producing microfilariae.
For a more complete evaluation of the Dirofilaria status in a dog or cat, it is recommended that an occult serological test and a microfilarial test be done at the same time. If both techniques are done the results may be interpreted as follows:
Antigen Positive and Microfilaria Negative
- single sex infection (female)
- immature adult worms (5-6 months postinfection)
- immune mediated clearance of microfilariae
- animal on monthly preventives, or after microfilaricide treatment
Antigen Negative and Microfilaria Positive
- microfilariae are not Dirofilaria immitis
- heartworm antigen not present, or present in levels too low to detect
- adult worms dead and antigen cleared, but microfilariae still present
- microfilarial contamination of lysing solution, dye or filter chamber
- animal transfused with microfilaremic blood
- prenatal transfer of microfilariae
- immune mediated clearance of antigen-antibody complexes
- antigen destroyed due to improper storage or treatment of sample
If both a microfilarial test and an occult serological test produce inconclusive results, then other diagnostic tests may be conducted, such as thoracic radiographs, electrocardiogram, blood chemistry, and urinalysis. If the animal is clinically normal, follow up testing in six months with an occult serological test is recommended if the initial testing is inconclusive. In a positive animal that has been treated, heartworm antigen can persist for up to four months. Repeat testing must be done after that time to confirm that treatment has been effective.
Nematode Larval Culture
| Category | Description |
|---|---|
| Test Code: | NLC |
| Test Name: | Nematode Larval Culture |
| Test Method: | Nematode larval identification |
| Sample Required: | 15-20 grams of feces |
| Collection container: | plastic leak proof container |
| Transport: | Unrefrigerated |
| Test Day: | M-F |
| Lag Time: | Two weeks |
| Species: | All species, particularly ruminants, camelids and horses |
| Results Format: | Identification of nematode larvae detected |
Interpretation
Most nematode eggs in the order Strongylida are not identifiable to genus and/or species when detected in a fecal sample. To resolve this, feces can be cultured to obtain third stage larvae which can then be identified further. This information is helpful in determining the species of “strongyle” nematodes present in an animal, and the treatment or management necessary to reduce or eliminate the worms.
The best sample for a Nematode Larval Culture is a freshly voided fecal sample that is not refrigerated and submitted for testing within two days of collection. Ground samples may contain free-living larvae that complicate the identification.
In this laboratory, this procedure is done only after a quantified double centrifugation concentration flotation procedure for parasites has been evaluated. If the number of “strongyle” eggs detected in this procedure is 100 per gram or more, the nematode culture can be done. If fewer than 100 eggs per gram are detected in the sample, enough larvae may not develop to allow representative identification of the parasite population.
Occult Heartworm Test
| Category | Description |
|---|---|
| Test Code: | OH |
| Test Name: | Occult Heartworm Test |
| Test Method: | Antigen capture enzyme-linked immunosorbent assay |
| Sample Required: | 1 ml of serum or plasma |
| Collection container: | red-top tube |
| Transport: | Ship on cold packs |
| Test Day: | Tuesday and Friday |
| Lag Time: | 1-7 days |
| Species: | Dog and Cat |
| Results Format: | Positive or Negative |
Interpretation
The Synbiotics DiroCHEK heartworm test is an indirect enzyme-linked immunosorbent assay (ELISA) used for the detection of adult female Dirofilaria immitis antigen in canine and feline serum or plasma. This test is validated for use in dogs and cats only. Other animals may be tested by this method, but reactivity in other species is not clearly defined. This test method can be used to detect Dirofilaria immitis five to six months after infection in dogs, and seven to eight months after infection in cats. Because of the nature of this disease in cats, veterinarians may want to consider including feline heartworm antibody testing for a more accurate assessment of a cat’s true infection status.
Antigen Positive
- single sex infection (female)
- immature adult worms (5-6 months postinfection)
- immune mediated clearance of microfilariae
Antigen Negative
- heartworm antigen not present, or present in levels too low to detect
- adult worms dead and antigen cleared, but microfilariae still present
- immune mediated clearance of antigen-antibody complexes
- antigen destroyed due to improper storage or treatment of sample
Parasite Identification
| Category | Description |
|---|---|
| Test Code: | PID |
| Test Name: | Parasite Identification |
| Test Method: | Identification based on characters of specimens |
| Sample Required: | Specimen for identification |
| Collection container: | plastic leak proof container |
| Transport: | Ship in 70% ethanol* or 10% formalin* |
| Test Day: | M-F |
| Lag Time: | 1-7 days |
| Species: | All species |
| Results Format: | Identification of specimen submitted |
Interpretation
Whole parasites - Specimens such as worms or arthropods that are found in or on animals can be submitted for identification. It is requested that worms be submitted in 70% alcohol* or 10% formalin*, and that arthropods be submitted in 70% alcohol* or in a container moistened with water* on gauze or paper towels.
Other samples that can be submitted for parasite identification include:
- Necropsy tissues, such as sections of organs and intestines with contents;
- Fluids, such as tracheal and cloacal washes, duodenal aspirates, urine, blood; Histological sections on slides;
- Free-living worms or arthropods people find on their animals, in their residences, in feed samples and environmental samples such as soil and composted material.
* For submissions of samples in vials containing less than 30 ml formalin or alcohol:
- Submit parasites for identification in a leak-proof and escape-proof plastic screw cap vial or jar.
- Add up to 29 ml (5 ml = approximately one teaspoon) of alcohol or formalin to the jar or vial, covering the parasite. Then the jar or vial must be placed in a leak-proof zipper-lock bag, also containing absorbent material sufficient to absorb the entire amount of liquid. If the shipment is made by ground delivery (USPS, FEDEX ground, or UPS ground) there are no special requirements for the vial or jar. If the shipment is to be made by air (USPS next day, FEDEX next day, UPS next day), the vials must be rated to withstand 95kPa internal pressure for air cargo.
- Print the label for UN3373 Biological Substance, Category B (do not reduce the size of the image for printing). Cut out label along the dotted line and affix to outer box with clear packing tape. Routine shippers can substitute commercially available peel-and-stick versions of this required label.
Sand Recovery from Horse Feces
| Category | Description |
|---|---|
| Test Code: | SAND |
| Test Name: | Sand Recovery from Horse Feces |
| Test Method: | Recovery of sand from feces |
| Sample Required: | 200 grams of feces |
| Collection container: | Plastic leak proof container |
| Transport: | Ship without cold packs |
| Test Day: | M-F |
| Lag Time: | 1-3 days |
| Species: | Horse |
| Results Format: | Sand Recovered, or Sand Not Recovered |
Interpretation
Consumption of sand and dirt by horses may be associated with diarrhea, weight loss, and colic due to irritation and obstruction of the gastrointestinal tract. The purpose of this test is to recover sand from fresh fecal samples to aid in the diagnosis of sand retention in horses. Horses maintained in sandy areas, fed on the ground, or that have a history of living in sandy areas may accumulate large amounts of sand in the gastrointestinal tract.
Results of fecal sand analysis may vary from sample to sample without treatment due to manure production, natural expulsion of the sand from the intestine, location of the sand in the intestine and manure consistency.
A minimum of 200 grams of dirt-free feces is needed for this test. This is approximately 8-10 fecal balls from a light horse breed, 6-7 fecal balls from a draft horse breed, and 12-14 fecal balls from a miniature horse.
The amount of sand will be reported as (based on a 200 gram sample):
No Sand Recovered or Sand Recovered
If sand is recovered from the fecal sample, this finding may/ may not correlate with the amount of sand that may be in the intestinal tract. However, if there is no recovery of sand, it does not rule out the presence of sand in the intestine. This test is only an aid in diagnosis of chronic diarrhea, weight loss and colic. The presence of any sand along with clinical signs may be a cause for concern and the need for treatment and management changes.
When clinical signs such as diarrhea or weight loss are present in any horse, it may be useful to submit enough feces (minimum of 250 grams) to do both a quantitative fecal flotation and sand analysis.
Skin Scrapings/KOH Digestion
| Category | Description |
|---|---|
| Test Code: | SSE |
| Test Name: | Skin Scraping KOH Digestion |
| Test Method: | Microscopic Examination and Digestion |
| Sample Required: | Representative sample of skin lesion |
| Collection container: | plastic escape proof container or ointment tin |
| Transport: | Ship on cold packs |
| Test Day: | M-F |
| Lag Time: | 1-4 days |
| Species: | All species |
| Results Format: | Identification of parasite recovered, or No Ectoparasites Detected |
Interpretation
Skin scrapings for ectoparasites must be submitted in an escape proof container such as a plastic container with a screw top or an ointment tin. All samples must be representative of the lesions on the animal.
Skin scrapings for the diagnosis of mange must be collected in a way that takes into account the nature of the lesion and the location of the mite causing the lesion.
Deep dwelling mites (such as Sarcoptes, Demodex) that cause minimal epidermal hyperplasia and lesions may be recovered by doing the following: dip a scalpel blade in mineral oil, pinch a fold of skin between the thumb and forefinger, hold blade at right angles to the skin, scrape until blood begins to seep from the abrasion, and place the scalpel blade with material removed into an escape proof container. Remember that unless you draw blood, you are not doing a good scraping!!!
Surface dwelling mites (such as Chorioptes) or lice that produce epidermal hyperplasia and exfoliation and lesions may be recovered by scraping the detritus into an ointment tin using the cover as a scraper, or by scraping the detritus with a scalpel blade into an escape proof container.
All skin scrapings are examined grossly using a dissecting microscope. If no arthropods are detected, the entire sample is put into a 5% potassium hydroxide solution and digested. This releases any arthropods that may be caught in tissue exudates or skin. The digested material is then prepared for microscopic examination and identification of any parasites detected.
Tritrichomonas foetus Identification
| Category | Description |
|---|---|
| Test Code: | TFE |
| Test Name: | Trichomonas Fecal Examination |
| Test Method: | InPouch™ TF Culture System |
| Sample Required: | Feces taken directly from cat’s rectum |
| Collection container: | InPouch™ TF Culture System (available from AHDC Shipping Department) |
| Transport: | Unrefrigerated and by overnight shipping |
| Test Day: | M-F |
| Lag Time: | Two weeks |
| Species: | Cats |
| Results Format: | Positive or Negative for Tritrichomonas |
Interpretation
This method is used for the diagnosis of Tritrichomonas foetus infection in cats. Since trichomonads do not form cysts and disintegrate quickly after leaving the host, they cannot be recovered from fecal samples in the same manner used for encysted protozoa and worm eggs. If Tritrichomonas is suspected, the feces must be submitted for examination in an InPouchTM TF culture system, which keeps the protozoa alive for identification in the laboratory. The InPouch system does not support survival of Giardia or Pentatrichomonas hominis trophozoites, so flagellated protozoa with the correct characters seen after the incubation period are considered to be Tritrichomonas foetus.


