Johne's Disease Program Sampling and Testing Guidance
Johne’s disease, or Pseudotuberculosis, is caused by the bacterium Mycobacterium avium subsp. paratuberculosis (MAP). The Animal Health Diagnostic Center (AHDC) participates in annual proficiency tests for Johne’s serology, culture, and PCR, and is approved to perform testing for the USDA Voluntary Johne’s Control Program. The New York State Department of Agriculture & Markets (NYSDAM) provides Johne’s testing subsidies for all cattle herds in New York State. Participants in the New York State Cattle Health Assurance Program (NYSCHAP) benefit from an additional Johne’s testing subsidy. Out-of-state Johne’s testing is offered at the full fee; please refer to the AHDC’s Test & Fee Schedule listings for specifics on individual tests. This document outlines the MAP testing options, followed by sampling guidance and testing rationale for various animal species. For testing and herd management consultation, veterinarians are encouraged to contact Veterinary Support Services at 607-253-3900 or vetsupport@cornell.edu.
| Species | Herd Testing Options | Individual Animal Testing Options |
|---|---|---|
Cattle Testing is typically not recommended for cattle under 18 mo. of age* (See Section B below for assistance with test selection and Section F below for test method discussion) | Johne’s Fecal PCR: feces
Johne’s Commercial ELISA: serum Whole herd testing. Suitable for testing Bos Taurus and Bos indicus breeds. Environmental – Johne’s PCR: manure 6 or more composite environmental samples from high traffic areas representing the entire herd of adult animals. (See Section E below for additional information regarding sample collection and handling.) | Johne’s Fecal PCR: feces Johne’s Individual Culture: tissue (GI), colostrum, semen, feces (with approval for specific cases/out of state requests) Johne’s Commercial ELISA: serum Not intended to be interpreted as an individual cow test. If utilized individually then PCR confirmation is recommended on feces. It should also be noted that there is a subset of animals that may be negative on this ELISA but still shedding MAP. Johne’s AGID: serum For testing clinical suspects only. Fecal PCR is a more definitive tests, however, and should typically be used on clinical suspects instead of serum antibody testing. |
Goats Testing is typically not recommended for goats under 6 mo. of age* (See Section B below for assistance with test selection and Section E below for test method discussion.) | Johne’s Fecal PCR, Caprine: feces Whole herd or subset testing. For NYS goat herd testing, this PCR test is the default fecal detection test.
Johne’s Commercial ELISA: serum Whole herd screening. Not intended to be interpreted as an individual goat test. If utilized individually then PCR confirmation is recommended on feces. It should also be noted that there is a subset of animals that may be negative on this ELISA but still shedding MAP. | Johne’s Fecal PCR, Caprine: feces No pooled PCR testing is available for goats. Pooling samples prior to submission is inappropriate. For NYS goat herd testing, this PCR test is the default fecal detection test. Johne’s Individual Culture: tissue (GI), feces (with approval for specific cases/out of state requests) Johne’s Commercial ELISA: serum Not intended to be interpreted as an individual goat test. If used individually then PCR confirmation is recommended on feces. It should also be noted that there is a subset of animals that may be ELISA negative but still shedding MAP. Johne’s AGID: serum For testing clinical suspects only. Fecal PCR is a more definitive tests, however, and should typically be used on clinical suspects instead of serum antibody testing. |
ALL Other Ruminants Sheep, Deer, Camelids, Exotics Testing is typically not recommended for other ruminants under 6 mo. of age* (See Section C below for assistance with test selection.) | Johne’s Individual Culture: feces Note: Fecal PCR testing has not been validated in our lab for any species other than bovine or caprine. All requests for Johne’s PCR on non-bovine, non-caprine species will receive Johne’s culture with Johne’s PCR. Johne’s Commercial ELISA: serum (for sheep) This test is for use on bovine serum or plasma and caprine serum. Ovine samples can be tested, but the assay has not been formally validated for use with samples from this species. This test is not used for testing camelids (e.g. alpaca, llama) or cervidae (deer, elk). Johne’s AGID: serum (for deer, camelids, exotics) Not available for sheep. For antibody detection in species for which no ELISA test is available. For cervidae and camelids, this test is used for surveillance in healthy animals and for clinical suspects. As cervidae are often infected with either or both the bovine and caprine strains of MAP, serum will be tested against both caprine and bovine reagents and incur double the cost unless requested otherwise. This test typically becomes positive late in the course of MAP infection. Culture with PCR testing is more definitive than serology. | Johne’s Individual Culture: feces, tissue (GI) Note: Fecal PCR testing has not been validated in our lab for any species other than bovine or caprine. All requests for Johne’s PCR on non-bovine, non-caprine species will receive Johne’s culture with Johne’s PCR. Johne’s Commercial ELISA: serum (for sheep) Ovine samples can be tested, but the assay has not been formally validated for use with samples from this species. This test is not used for testing camelids (e.g. alpaca, llama) or cervidae (deer, elk). Johne’s AGID: serum (for deer, camelids, exotics) Not available for sheep. For antibody detection in species for which no ELISA test is available. For cervidae and camelids, this test is used for surveillance in healthy animals and for clinical suspects. As cervidae are often infected with either or both the bovine and caprine strains of MAP, serum will be tested against both caprine and bovine reagents and incur double the cost unless requested otherwise. This test typically becomes positive late in the course of MAP infection. Culture with PCR testing is more definitive than serology. |
* Age recommendations related to Johne’s disease test applications are made based upon the typical expected onset of clinical disease in a particular species. Very heavy environmental contamination or management factors associated with high infectious doses or repeated/continuous exposure may rarely lead to very early onset of clinical disease. This is unusual in a population or facility that has never previously detected Johne's disease. In addition, there is a delay in seroconversion after infection detected by ELISA and other current serology tests available. Additionally, animals under 3-4 months of age may have detectable maternal antibodies and may or may not be infected.
A. Preparing samples for Johne’s Testing
- The animal’s identification and submission sample number must be written on all blood tubes and fecal containers with a waterproof marker.
- Circle the sample number on the container to distinguish it from the animal’s ID.
- On the accession form, please specify the exact name of the testing option from the table above, as more than one test can be performed on a single sample type.
- Use same animal ID from test to test to track progress of animals over time.
- For proper test interpretation, a brief Johne’s history must be provided with date of birth and species tested.
- For forms and information, call Regulatory affairs at 607-253-3938. For sample containers and mailers, contact shipping at 607-253-3935 or see the supplies page. We recommend use of the plastic screw-capped container available for fecal samples from our shipping department.
Johne’s Fecal PCR/Pooled PCR and Johne’s Cultures
- Use a clean, plastic sleeve to collect each sample to avoid cross-contamination. Feces must be transferred to an appropriate clean container. Containers should be wide mouthed, unbreakable screw-capped plastic jars that can withstand internal pressures associated with shipping and bacterial fermentation. Recommended fecal containers may be purchased from the laboratory (supplies page; 607-253-3935; ahdlshipping@cornell.edu).
- Fill container 1/2 full with feces taken directly from rectum, and seal tightly. Note that lubricant does not inhibit PCR or culture testing. Samples that are liquid should be placed in the fecal cup and then placed individually in a zipper-lock bag. At least 10 grams (3 tsp) of feces is needed. Containers that are over half-full or have loose lids can leak, cause cross-contamination, and may be rejected at the laboratory. Please do not use plastic sleeves or gloves as the primary container. Additional fees ($50/hr.) are incurred if samples require cleaning, transferring to appropriate containers, or excessive handling to process.
- Put no more than 25 containers in a large leak-proof zipper-lock plastic bag; then place one or more bags in an insulated shipping container with cold packs. Include absorbent material inside each zipper-lock bag. Ship with UN 3373, Biological Substance, Category B Label on outside of box.
Occasionally fungal and bacterial contaminants overgrow fecal samples. Contamination might be linked to feeds or environment of particular farms. To minimize contamination, fecal samples should be taken and shipped promptly (with cold packs). Do not freeze samples unless they cannot be submitted within 48 hours of collection, then they should be frozen at -20C (chest freezer) to reduce mold over-growth. Avoid storing in freezer portion of a refrigerator.
- For Johne’s pooled PCR testing, individual animal samples must be submitted as above. Pooling will be performed at the laboratory. Do not pool samples prior to submission. If a pool is positive, individual PCR testing must be performed to determine which animal(s) in the pool was positive. This will incur additional fees.
Johne’s PCR – Environmental
See Section E, below, for a complete discussion on environmental sampling
Johne’s Commercial ELISA
- Test is performed on serum samples. Please submit 2 mL of serum.
- Johne’s commercial ELISA results will be available 2-7 days after submission of serum samples.
- Confirmation of positive ELISA results by Direct Fecal PCR on feces is strongly recommended. Follow ALL submission criteria for fecal samples as stated above.
Johne’s AGID
- Test is performed on serum samples. Please submit 1-2 mL of serum.
B. Rationale for Selection of Tests for Cattle
The recommended test or test combinations depend upon the specific farm situation. Review the herd situation thoroughly with the herd managers. Determine the probable extent and impact of Johne's disease in the herd, farm goals, practices predisposing to spread of infection, and the client's goal and resources for Johne's control. Veterinarians may call 607-253-3900 to arrange consultation with a veterinary support veterinarian on an optimal test regimen.
The expected herd prevalence of infection plays an important role in selection and interpretation of tests for Johne’s disease and in the actions taken based upon test results. Management strategies, including testing choices, are typically different for dairy herds and beef herds. Since the young stock remain suckling on their dams until weaning in a typical cow/calf herd, exposure to adult manure raises the transmission risk throughout the suckling period. Management of MAP infection in dairy herds sufficient to reduce transmission to young animals may be achievable with less stringent testing than might be necessary in cow/calf populations.
About 90% or more of long-standing infections with clinical disease are detectable by testing. Early infections, however, do not necessarily stimulate antibody production, and shedding of organisms may be intermittent; thus, fewer than 45% of such animals are detectable by culture (< 20% by serology) at a single point in time. Repeated testing over time enhances detection of sub-clinically infected animals and will increase assurance that a herd is low risk for MAP infection.
Our serological test to screen for MAP infection in non-clinical cattle is the commercial ELISA kit from VMRD. The kit states 93.1% sensitivity and 90.0% specificity for bovine serum and 84.4% sensitivity and 82.1% specificity for bovine milk, however false-positive results can occur from exposure to other cross-reacting environmental mycobacteria. For this reason, antibody testing is considered a presumptive test at the herd or individual animal level. Confirmation of antibody positive results by fecal PCR is strongly recommended. A subset of animals may test negative on this ELISA but still shed MAP. For this reason, a combination of fecal detection testing by PCR and serologic testing by ELISA is typically recommended for management strategies in cow/calf herds.
Selection of Tests for Bovine Herd Testing
Testing is most useful at the herd—and not the individual animal—level. Johne’s Fecal PCR, Johne’s Pooled Fecal PCR, and the Johne’s Commercial ELISA test can be used in various combinations, with the most information for detecting MAP infection coming at the greatest cost. Herd testing options, from highest to lowest quality of information and cost, are as follows:
- Johne’s Fecal PCR plus Johne’s commercial ELISA on all animals—the most aggressive and costly method. May be useful in herds where the goal is complete eradication and removal of infected non-shedders is desired. This combination of tests may be necessary to employ in cow/calf herds, especially herds of genetically valuable seed stock, to reduce the risk of undetected shedding to the absolute minimum possible when young animals are commingled with adults, especially prior to weaning.
- Johne’s Fecal PCR ONLY on all animals.
- Johne’s Pooled Fecal PCR (pools of 5 created in the lab from individual samples submitted); only economical in low prevalence herds as all positive pools will have each individual sample in the pool tested by PCR, with additional fees incurred.
- Johne’s Commercial ELISA on all animals first, with Johne’s Fecal PCR follow-up on individual animals with positive values. In herds of unknown history, we recommend using this strategy to initially screen a herd for Johne’s infection. It should be noted that there is a subset of animals that may be negative on the ELISA but still shedding MAP.
- Only Johne’s Commercial ELISA on all animals for estimation of herd prevalence of Johne’s infection (may not be accurate in some herds with cross-reacting bacteria).
Testing of Individual Cattle
As described above, no single test will detect all infections because animals respond differently at various stages of infection. Tests generally work best in individual animals with advanced MAP infection showing clinical signs; 85-90% of such cases have antibody and close to 100% will be shedding high numbers of MAP. However, 10-15% of clinical cases do not have an antibody response. Animals with early, subclinical infection intermittently shed organisms in their feces and < 20% have an antibody response at this stage of infection. Therefore, test results for individual animals must be interpreted with caution. Negative test results do not necessarily mean that an animal is not infected. As with herd testing, repeated testing of an individual animal over time provides more assurance of a lower risk status for Johne’s infection.
- High sensitivity, rapid turnaround, and reasonable fees make the Johne’s fecal PCR the test of choice for clinical suspects.
- If feces cannot be obtained, Johne’s AGID would be the recommended serologic test for clinical suspects. (see Section F, below, for more information on the AGID). Since this test becomes positive later in the course of infection, it will miss early infections. Rarely, immunosuppression associated with advanced chronic diseases such as Johne’s disease will cause the infected bovine to become non-reactive immunologically to MAP infection. So, serum testing in a subset of clinical animals will yield a false negative result.
- Histopathology: A presumptive diagnosis of Johne’s disease can be made with compatible histopathology, including acid-fast staining of tissues. Feces or colon contents can be tested by Johne’s Fecal PCR to confirm the diagnosis. Also, tissues from the distal ileum, ileocecal junction and associated lymph nodes, and mesenteric lymph nodes can be submitted for Johne’s Culture to confirm the diagnosis.
Export and Regulatory Testing
It is important to determine which test will be accepted by the buyer or governmental oversight agency to satisfy animal movement testing needs.
C. Rationale for Selection of Tests for Goats
The recommended test or test combinations depend upon the specific farm situation, including farm economics and goals. Review the herd situation thoroughly with the herd managers. Determine the probable extent and impact of Johne’s disease in the herd, farm goals, practices predisposing to spread of infection, and the client's goal and resources for Johne’s control. Veterinarians may call 607-253-3900 to arrange consultation with a veterinary support veterinarian on an optimal test regimen. The expected herd prevalence of infection plays an important role in selection and interpretation of tests for Johne’s disease and in the actions taken based upon test results. Management strategies, including testing choices, are typically different for dairy goats and meat goats, due to the differences in management of the young stock, which varies from complete separation at birth from the dams, to suckling on the dams for the entire pre-weaning period.
About 90% or more of long-standing infections with clinical disease are detectable by testing. Early infections, however, do not necessarily stimulate antibody production, and shedding of organisms may be intermittent; thus, fewer than 45% of such animals are detectable by culture (< 20% by serology) at a single point in time. Repeated testing over time enhances detection of sub-clinically infected animals and will increase assurance that a herd is low risk for MAP infection. Clinical disease due to MAP infection has been reported as early as 6 months of age in goats. Maternal antibodies may complicate interpretation of serum antibody tests prior to 4-6 months of age.
Our recommended test for screening for MAP infection in clinical or non-clinical goats is the Johne’s fecal PCR test. It is highly sensitive and specific in shedding animals but may result in false negatives during early infection prior to the onset of shedding or during intermittent shedding periods.
Our recommended serology screening test is the Johne’s commercial ELISA test. The VMRD MAP ELISA kit used at the AHDC states 100% sensitivity and 98.5% specificity for caprine serum, however false-positive results can occur from exposure to other cross-reacting environmental mycobacteria. For this reason, serological testing is considered a presumptive test at the herd or individual animal level. Confirmation of seroreactors by fecal PCR is strongly recommended. It should also be noted that there is a subset of animals that may be negative on this ELISA but still shedding MAP. For this reason, a combination of fecal detection testing by PCR and serologic testing by ELISA is typically recommended for management strategies in herds with kids suckling on their dams.
Selection of Tests for Goat Herd Testing
Testing is most useful at the herd—and not the individual animal—level. Johne’s Fecal PCR, Caprine, and the Johne’s Commercial ELISA tests can be used in combination or separately, with the most information for detecting MAP infection coming at the greatest cost. Pooled Johne’s fecal PCR is not available in goats. Herd testing options, from highest to lowest quality of information and cost, are as follows:
- Johne’s Fecal PCR, Caprine plus Johne’s Commercial ELISA on all animals – the most aggressive and costly method. May be useful in herds where the goal is complete eradication and removal of infected non-shedders is desired. This combination of tests may be necessary to employ in herds in which kids are allowed to suckle their dams until weaning, especially herds of genetically valuable seed stock, in order to reduce the risk of undetected shedding to the absolute minimum possible when young animals are commingled with adults, especially prior to weaning.
- Johne’s Fecal PCR, Caprine ONLY on all animals.
- Johne’s Commercial ELISA on all animals first, with Johne’s Fecal PCR, Caprine follow-up on individual animals with positive values. In herds of unknown history, we recommend using this strategy to initially screen a herd for Johne’s infection. It should be noted that there is a subset of animals that may be negative on the ELISA but still shedding MAP.
- Only Johne’s Commercial ELISA on all animals for estimation of herd prevalence of Johne’s infection (may not be accurate in some herds with cross-reacting bacteria).
D. Rationale for Selection of Tests for Sheep, Deer, Camelids, and Exotic Ruminants
Culture: Strain variants of MAP have been identified in sheep, bison, and occasionally in goats. Sheep, goats, farmed cervids, and camelids can also be infected with the bovine strain type. The strain variants are more fastidious than the bovine strain type and require different culture methods and longer incubations periods. Johne’s cultures are routinely held for 10 weeks on sheep samples, and for 8 weeks on all other non-bovine species. Due to the intracellular nature of MAP, tissue culture of the ileocecal junction (alternatively distal ileum and/or mesenteric lymph nodes) is recommended on necropsy samples, in conjunction with histopathology and acid-fast staining.
As in cattle, serologic tests for Johne's disease generally detect individuals in later stages of infection. Approximately 10% of clinical animals are seronegative and on average, serology will detect 20-30% of infections; therefore, a negative test does not always indicate freedom from infection in the individual animal or at the herd level. Repeated testing provides a more accurate assessment of infection status of individuals or herds. Cross-reactions can occasionally cause false positive seroreactions. Culture and/or histopathology with tissue culture is considered definitive and is recommended for confirmation in flocks or herds with elevated serology. The Johne’s AGID is the default serology test for camelids, cervidae and other exotic ruminants for both surveillance and clinical suspects. The Johne’s Commercial ELISA is performed on sheep serum; however, the test is not validated for this species.
Note: A finding of repeat AGID positives in herds with compatible pathology but negative fecal cultures warrant further investigation into the possibility of infection by an unculturable strain variant of MAP.
Selection of Tests for Ruminants other than Cattle or Goats
Testing is most useful at the herd —and not the individual animal —level. Johne’s Culture, and the Johne’s Commercial ELISA or AGID tests can be used alone or in combination, with the most information for detecting MAP infection coming at the greatest cost. Johne’s Fecal PCR is only validated in cattle and goats; therefore, Johne’s Culture is performed on all other species. Herd testing options, from highest to lowest quality of information and cost, are as follows:
- Johne’s Culture, plus Johne’s Commercial ELISA (sheep) or Johne’s AGID (camelid, cervid, exotic bovidae) on all animals – the most aggressive and costly method. (Note: the ELISA will not be performed on samples from camelids, cervids, or exotic bovidae.) May be useful in herds and valuable animal collections such as zoos where the goal is complete eradication and removal of infected non-shedders is desired. This combination of tests may be necessary to employ in herds in which young stock are allowed to suckle their dams until weaning, in order to reduce the risk of undetected shedding to the absolute minimum possible when young animals are commingled with adults, especially prior to weaning. Since the PCR assay is not validated in non-bovine, non-caprine species, the culture is performed as the gold standard test methodology. However, the longer lag time to get back a culture favors the use of PCR testing to supplement culture when more rapid detection, especially of clinical suspects, is desired. The non-validated Johne’s Fecal PCR, Caprine can be requested with the Johne’s Culture in these scenarios.
- Only Johne’s Culture, +/- Johne’s Fecal PCR, Caprine on all animals. Note: this PCR can be performed on non-bovine, non-caprine species with a disclaimer that it is not validated in that species and must be run in conjunction with Johne’s culture. If the manure sample is small, Johne’s culture will be prioritized over PCR.
- Johne’s Commercial ELISA (sheep) or Johne’s AGID (camelid, cervid, exotic bovidae) on all animals first, with Johne’s Individual Culture follow-up of animals with positive values. In herds of unknown history, we recommend using this strategy to initially screen a herd for Johne’s infection. It should be noted that there is a subset of animals that may be negative on the ELISA but still shedding MAP.
- Only Johne’s Commercial ELISA (sheep) or Johne’s AGID(camelid, cervid, exotic bovidae) on all animals for estimation of herd prevalence of Johne’s infection (may not be accurate in some herds with cross-reacting bacteria).
Note: Histopathology with acid-fast staining of tissues from the ileocecal junction, distal ileum, and the mesenteric lymph nodes, and culture of these tissues are also considered diagnostic for MAP. This is suitable for confirming lesions consistent with Johne’s disease in clinically suspect mortalities of any species.
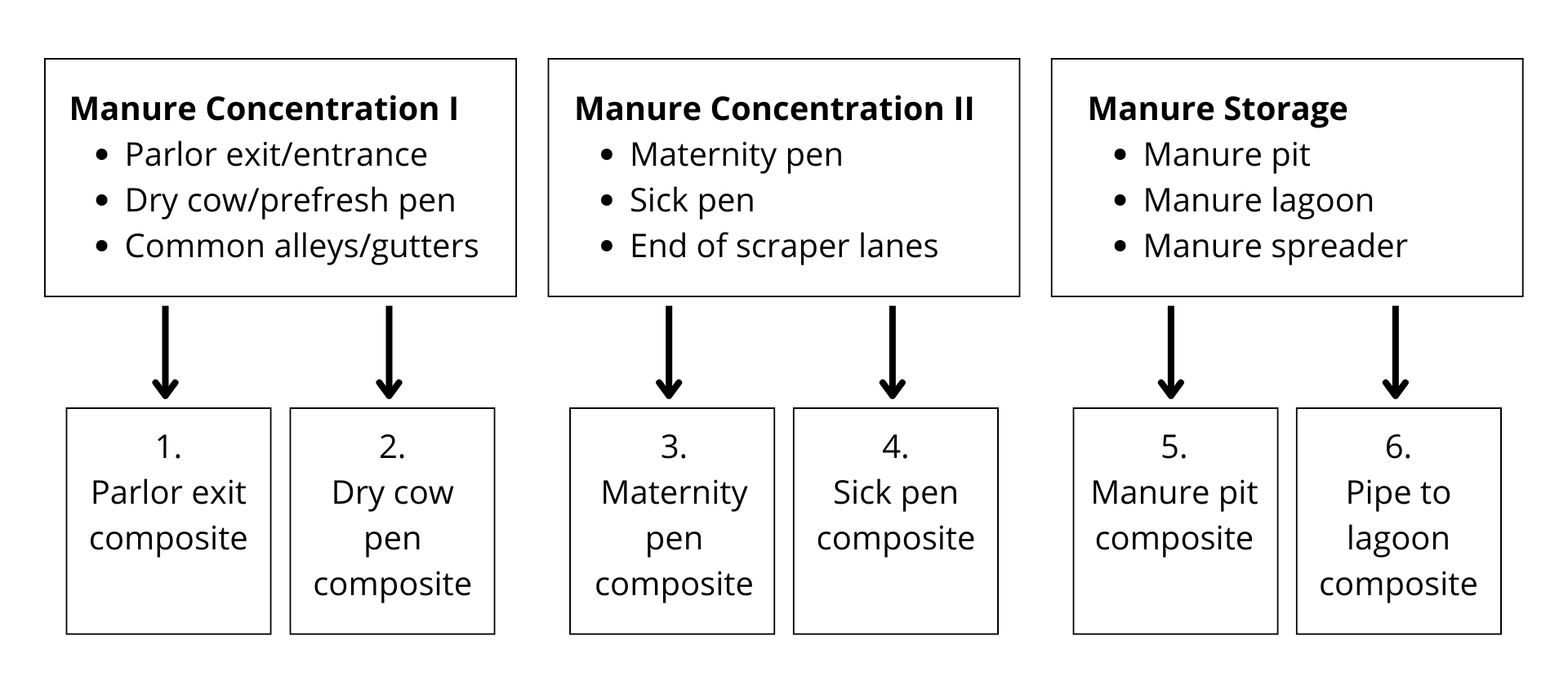
E. Strategy and Instructions for Environmental Sampling
Sample submission protocols recommended by the Animal Health Diagnostic Center
Note: Six composite samples are required (as shown in the above example) for New York State subsidized prices to apply. Four of the six samples should come from common cow areas (boxes 1 and 2) that represent the entire adult herd present at the point in time of testing. (see point 2 below under Dairy Herd Johne’s Environmental Testing Strategy).
Each composite sample should consist of four (4) ~5 gram subsamples, for a total of 20 grams per location. Add the subsamples to a clean container or disposable zipper lock bag to mix the composite well. Place ~ 10 grams of the mixed composite sample into a standard Johne’s fecal cup (approximately ½ full) to submit to the AHDC for testing. Do not overfill the cup. Use a clean glove to sample each different location to avoid cross-contamination.
If you are using the federal guidelines for sampling lagoons, please do not submit gauze squares to the laboratory. Instead, place gauze with feces in the mixing container as previously described, mix well and submit feces only in the standard fecal cup. Manure storage samples can also be obtained by using a clean cup fixed to a long pole.1 Please use safety precautions!
Laboratory Paperwork
NYSCHAP form must be used for NYSCHAP herds to receive subsidized testing.
- Complete sets of six samples are required for NYSCHAP or NYS subsidized testing
- ID column – Please identify the specific location sampled for each of the six composite samples
- AHDC Accession Form – in species and breed columns – to identify farm operation type state (Bovine) and (beef or dairy) respectively in the species and breed columns. On the NYSCHAP form, indicate beef or dairy in the breed column and bovine in the comment column.
- Sample type should be listed as manure (= manure from the adult cow environment, not directly from cows).
- Test requested should be Johne’s Fecal PCR.
- Please specify Johne’s environmental testing strategy in the history section and indicate if strategy is used for USDA Test Negative Status Program or herd monitoring.
Dairy Herd Johne’s Environmental Testing Strategy
- USDA Test Negative Status Program: Annual environmental cultures can be used as one testing option for Dairy herds to achieve Level 1 or to maintain monitored status of testing levels already achieved. Samples should include composites representing the entire adult herd including dry cows. See link at USDA Johne's Control Program, found on the USDA APHIS Johne's website, under "APHIS' Response."
- NYSCHAP and NY Program Recommendation: The objective of environmental sampling in this protocol is to screen a herd for MAP infection or to monitor MAP infection loads in known infected herds. For monitoring herds of unknown status, a minimum of twice annual sampling ~ 6 months apart is recommended. Complete sets of six samples are required for NYSCHAP or NYS subsidized testing. Samples from common cow alleys (including dry cows) should represent a commingled sample of all adult cows in the herd on the day of sampling. Sampling large manure storage areas such as lagoons can be complicated because storage samples may represent a commingled sample (manure and sometimes milkhouse waste) collected over a longer period of time with dilution effects and declining MAP survival over time. When possible, sampling from or near common entry pipes delivering fresh manure to the lagoon or tank from the adult barns without milkhouse waste can reduce some of the dilution and survival issues. For additional examples, see USDA Johne's Control Program, found on the USDA APHIS Johne's website, under "APHIS' Response."
Recommendations for Sampling Beef Herds
- Herd monitoring only: Johne’s Environmental Testing is not currently recognized by USDA for the Johne’s Test Negative Status Program in beef herds.
- Complete sets of six samples are required for NYSCHAP or NYS subsidized testing.
- Spring and Fall sampling time frames are recommended.
- Recommended Locations: Common sites that represent the adult cattle (including bulls) in the herd.
- Pre-fresh and Calving pens or paddocks, Sick Pen (if separate)
- Common lanes or alleys including the chute when the herd is being handled
- Around congregation points on pasture including:
- Feed troughs, Hay manger
- Water troughs
- Mineral or supplement feeding stations
Note: No environmental testing strategies have been validated for use in screening/surveillance efforts for detection, management or control of MAP infection in non-bovine species.
F. Brief Description of Test Methods Used in the Johne’s Program
Johne’s Fecal Culture
The Laboratory uses a liquid media culture system for all samples. Bovine cultures are incubated for 35 days. For sheep, cultures are incubated for a maximum of 70 days. For goats, cervids, camelids, Bovidae and other exotic species, cultures are incubated 56 days.
All cultures are checked by acid-fast staining at times throughout incubation and once the testing is complete. Bottles with acid-fast organisms detected are sent for PCR confirmation of MAP. Semi-quantitative results are provided on all positive samples based on days to positive in culture once confirmed. Negative samples have final results available in approximately 35-42 days for domestic cattle, 70-77 days for sheep, and 56-63 days for all other species.
Johne’s Fecal PCR
The Johne’s Fecal PCR test is a real-time polymerase chain reaction assay with an internal amplification standard control for every sample. It is validated for high throughput in a 96-well plate format. It detects amplified DNA of MAP extracted from fecal samples of shedding ruminants. PCR assays are run in thermal cyclers, which produce results as “Detected” with the numbers of cycles to positive (Ct) versus “Not Detected”, and rarely "Inconclusive"*. Validation of this assay included a quantitative evaluation of the relationship between cycles to positive and the number of colony forming units (CFU) on solid HEY agar culture, which has been used as the gold standard for MAP detection. Since a statistically significant relationship exists between Ct and CFU, a quantitative result will also be reported, suggesting shedding at various levels. PCR Ct values for bovine and caprine fecal samples will include an interpretation at the bottom of the results.
All PCR results will be reported as “Detected”, “Not Detected” or “Inconclusive”, with a Ct value which can be interpreted as follows:
Bovine
| Ct Value | Interpretation |
|---|---|
| Less than 30.9 | Heavy shedder |
| Greater than or equal to 30.9 and less than 34.4 | Moderate shedder |
| Greater than or equal to 34.4 | Light shedder |
Caprine
| Ct Value | Interpretation |
|---|---|
| Less than 25.2 | Heavy shedder |
| Greater than or equal to 25.2 | Positive, Not Heavy shedder |
* Rarely, samples are reported as “Inconclusive” when something in the fecal sample inhibits the ability of the PCR assay to detect the target (MAP bacteria) and would result in a false negative test result.
PCR results on any non-bovine/non-caprine specimens will be reported with a disclaimer, as this assay has not been validated yet for any other species.
Johne’s Fecal PCR testing is one of the approved methods for testing in the National Voluntary Johne’s Test Negative Status Program (TNSP) cattle herds. More information can be found in the USDA Johne's Control Program standards, found on the USDA APHIS Johne's website, under "APHIS' Response."
Johne’s Pooled Fecal PCR
Fecal PCR testing (as described above) performed on in-house pooled bovine samples only. Individual fecal samples should be submitted to the laboratory using standard Johne’s fecal sampling practices. Please specify Johne’s Pooled Fecal PCR on the accession form as the test requested. The laboratory routinely pools 5 samples. During laboratory accessioning, leftovers of 3 to 4 samples will be pooled. Leftovers of 1 or 2 samples will be tested individually by PCR. The pooling of samples will be done in the lab, and the related individual samples will be catalogued and frozen for further testing if necessary. You will not have to re-submit samples.
Individual samples from positive pools will automatically be set for individual PCR and appropriate charges for the individual PCRs will be applied to the accession. Consequently, pooled PCR results take 7-10 business days, with individual PCR results from positive pools expected in an additional 7-10 business days.
Note: Clinical suspects or high-risk samples should not be tested in pools. As fecal shedding exceeds 10% of the herd and approaches 20%, the tendency will be for most or all pools to be positive. Therefore, herds with 10% or more expected shedding should avoid Pooled PCR testing.
Johne’s Commercial ELISA
The AHDC utilizes a USDA-licensed Johne’s commercial enzyme-linked immunosorbent assay (ELISA) test to detect antibodies directed against MAP in bovine serum. As with all Johne’s serology, the ELISA is intended to be interpreted primarily at the herd or group level rather than at the individual animal level. The ELISA can be a useful tool to monitor herd prevalence of Johne’s disease and to assess the prevalence of source farms for purchased replacements. Potentially, the ELISA could also be used as an adjunct in herds where the goal is complete eradication, and the removal of infected non-shedders is desired.
The results of the Johne’s Commercial ELISA will be reported as “Positive” or “Negative” with a numerical corrected optical density value assigned that will allow for comparison of results over time. Since the concern of most farms is the detection of cattle that are shedding MAP, and therefore infectious, it is recommended that this serology test be used in conjunction with antigen detection tests on feces such as Fecal PCR prior to culling animals. It should also be noted that there is a subset of animals that may be negative on this ELISA but still shedding MAP.
Johne’s AGID
Agar gel immunodiffusion (AGID) is another antibody detection methodology routinely employed for detecting antibodies against MAP. Results are reported as “Positive”, “Negative”, or “Inconclusive”, based on lines of precipitin forming in an agar matrix between antigen and serum sample wells. Published specificity of AGID tests are 99-100%, as compared to ELISA test specificities from 98.2-99.5%2,3. The test sensitivity is expected to be relatively high (>/= 80%) for clinical suspects2,3, and similar to or less than ELISA assays for subclinical animals (8.0-56%)4,5. This supports using AGID tests as the most suitable serological test for clinical suspects in the absence of MAP detection by culture, PCR, or histopathology.
Studies in sheep herds with caseous lymphadenitis, but without paratuberculosis, have shown cross-reacting antibodies with Corynebacterium pseudotuberculosis for some ELISA tests, though not for AGID tests, reducing the specificity of ELISAs to 64%5. The AGID may therefore be useful in some control efforts, especially for small ruminants and cervidae, particularly where Johne’s culture or PCR assays are either not available or beyond the economic resources for the herd. Currently, reagents are not available for sheep AGID testing.
Sale Testing
See comments for individual animal testing. Current testing provides limited accuracy in defining an individual animal’s Johne’s infection status.
References
- Raizman, Wells, et al. The Distribution of Mycobacterium avium ssp. Paratuberculosis in the Environment Surrounding Minnesota Dairy Farms. J. Dairy Sci. (2004) 87:2959-2966.
- Dubash K, Shulaw WP, Bech-Nielsen S, et al. Evaluation of an agar gel immunodiffusion test kit for detection of antibodies to Mycobacterium paratuberculosis in sheep. JAVMA (1996) 208 (3):401.
- Dubash K, Shulaw WP, Bech-Nielsen S, et al. Evaluation of an enzyme-linked immunosorbent assay licensed by the USDA for use in cattle for diagnosis of ovine paratuberculosis. J Vet Diag Invest (1995) 7:347
- Hope AF, Kluver PF, Jones SL, Condron RJ Sensitivity and specificity of two serological tests for the detection of ovine paratuberculosis. Aust Vet J (2000) 78 (12): 850-6.
- Robbe-Austerman S, et al. Sensitivity and specificity of the agar-gel-immunodiffusion test, ELISA and the skin test for detection of paratuberculosis in the United States Midwest sheep populations. Vet Res 37 (2006): 553-64.
- Scott HM, Fosgate GT, Libal MC, Sneed LW, Erol E, Angulo AB, Jordan ER. Field testing of an enhanced direct-fecal polymerase chain reaction procedure, bacterial culture of feces, and a serum enzyme- linked immunosorbent assay for detecting Mycobacterium avium subsp paratuberculosis infection in adult dairy cattle. Am J Vet Res. 2007 Mar;68(3):236-45
- Clark DL Jr, Koziczkowski JJ, Radcliff RP, Carlson RA, Ellingson JL. Detection of Mycobacterium avium subspecies paratuberculosis: comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction. J Dairy Sci. 2008 Jul;91(7):2620-7.
- Alinovi CA, Ward MP, Lin TL, Moore GE, Wu CC. Real-time PCR, compared to liquid and solid culture media and ELISA, for the detection of Mycobacterium avium ssp. paratuberculosis. Vet Microbiol. 2009 Apr 14;136(1-2):177-9.
United States Department of Agriculture, Animal and Plant Health Inspection Service. Uniform program standards for the voluntary bovine Johne’s disease control program [Internet]. Washington, DC: USDA APHIS; 2010 [cited 2025 Aug 9]. Available from the USDA APHIS Johne's website, under "APHIS' Response."
OPS VSS-WEB-1008-V02