Equine Cytokine 5-plex Assay
Cytokines are soluble messenger molecules of the immune system. They are indicators of innate and adaptive immunity and have multiple stimulating and regulatory functions during immune responses1-3. Individual cytokines are indicative for certain immune responses and can be used to access the immune status during health and disease. Cytokine detection in diagnostic settings for assessing clinical samples can be used to understand their contribution to disease, pathology, protection, immune activation or suppression and overall to evaluate the health status of a horse.
The Horse Cytokine 5-plex Assay detects IL-4, IL-10, IL-17, IFN-γ and IFN-α in equine samples (see below). The assay can thus be used to identify typical T-cells and anti-viral cytokines.
- IL-4 is a typical cytokine of the T-helper 2 (Th2) response and induces B-cell maturation and antibody production. However, it can also be produced by other cell types such as basophils, mast cells or eosinophils.
- IL-10 is produced by most regulatory T-cells (Tregs). Many other cell types of the innate and adaptive immune response also secrete IL-10 which functions as a regulatory and anti-inflammatory cytokine.
- IL-17 is made by Th17 cells and is an inflammatory cytokine. Although its role in equine immunity is less well known, IL-17 is likely involved in many inflammatory processes and autoimmune conditions.
- IFN-γ is the most important cytokine of the Th1 response and supports the induction of cellular immunity to defend intracellular pathogens. IFN-γ can also be produced by other cytotoxic Tcells, natural killer cells and some macrophages.
- IFN-α is an anti-viral cytokine and is often secreted in response to viral infection. It can be produced by almost all cells. However, specific cells called ‘plasmacytoid dendritic cells’ in the peripheral blood have been shown to be major producers of IFN-α. IFN-α is a major inducer of cytotoxic T-cells which are involved in the defense of intracellular pathogens.
Principle of the Cytokine 5-plex Assay
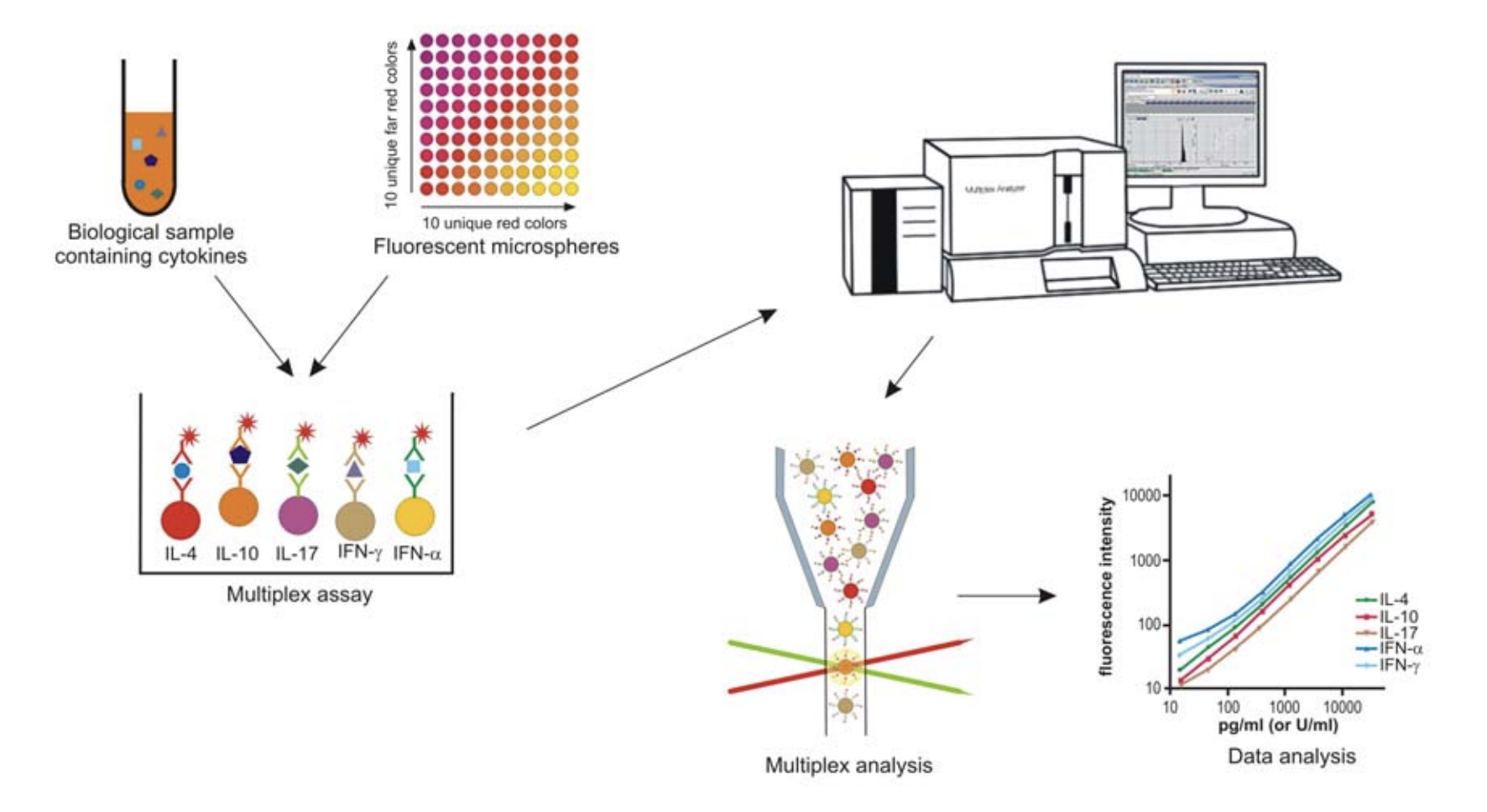
Multiplex assays use the principle of simultaneous detection of soluble analytes, such as cytokines, in biological samples4, 5. The Horse Cytokine 5-plex assay is based on fluorescent beads (‘microspheres’) that are color-coded and can be distinguished during the analysis of the assay6. Each cytokine bead assay uses a pair of monoclonal antibodies that are specific for the respective equine cytokine. This results in simultaneous measurements for five individual cytokines within the same sample (Fig. 1).
Limits of detection
The analytical sensitivities (lower limit of detection) and the upper limits of detection for cytokines in the 5-plex assay are shown in Table 1. The sensitivity of cytokine detection by the multiplex assay is increased by 13 to 150-fold compared to corresponding ELISAs6. This allows the measurement of equine cytokines in the 5-plex assay within their physiological ranges.
| IL-4 (pg/ml) | IL-10 (pg/ml) | IL-17 (U/ml) | IFN-γ (U/ml) | IFN-α (pg/ml) | |
|---|---|---|---|---|---|
| Lower limit of detection | 40 | 15 | 10 | 10 | 12 |
| Upper limit of detection | 80000 | 35000 | 10000 | 5000 | 30000 |
Samples for cytokine analysis
Cytokines are easily accessible in different body secretions and their measurement has a wide variety of potential applications during infectious diseases and inflammatory conditions. Equine samples that have been tested and validated at the AHDC include serum, plasma, colostrum, CSF, vitreous fluid, and nasal secretions. In addition, cytokine detection was validated for supernatants from in vitro cultures of equine cells. It is likely that many other equine body fluids can also be analyzed with the assay. If your sample is not included in the upper list, please contact the Serology Lab before submission.
Sample submission
For cytokine detection, 1 ml serum, colostrum, CSF, 0.5ml for other body fluids, or 0.5ml of cell culture supernatant should be submitted. Serum or other body fluids should be collected in a red top blood tube. The sample should be kept refrigerated and shipped by overnight shipment on an ice pack to the Animal Health Diagnostic Center at Cornell University. For more information, please see the submission page.
Samples are tested Monday and Thursday. Please contact the Serology/Immunology laboratory at the Animal Health Diagnostic Center at Cornell University before a larger sample submission is planned for this assay to provide us with approximate sample numbers and to ensure the timely measurement of your samples.
References
- Mosmann TR, Coffman RL. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7: 145-173.
- Moore, K.W., de Waal Malefyt, R., Coffman, R.L., O'Garra, A., 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683-765.
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. 2007. Interleukins 1β and 6 nut not transforming growth factor-β are essential for the differentiation of interleukin-17 producing human T helper cells. Nat. Immunol. 8: 630-638.
- Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. 2004. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin. Immunol. 110: 252-266.
- Prabhakar U, Eirikis E, Reddy M, Silvestro E, Spitz S, Pendley C, Davis HM, Miller BE. 2004. Validation and comparative analysis of a multiplexed assay for the simultaneous quantitative measurement of Th1/Th2 cytokines in human serum and human peripheral blood mononuclear cell culture supernatants. J. Immunol. Meth. 291: 27-38.
- Wagner B, Freer H. 2009. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol 2009; 127: 242-248.12


