Lyme Disease Multiplex Testing for Horses
Background
Lyme disease is induced by the spirochete B. burgdorferi. Spirochetes are transmitted to horses by infected ticks. Similar to humans, horses are incidental, dead-end hosts for B. burgdorferi1. Not all infected horses develop clinical signs of Lyme disease. If clinical signs occur, they can include chronic weight loss, sporadic lameness, shifting leg lameness, low-grade fever, muscle tenderness, chronically poor performance, swollen joints, arthritis and diverse orthopedic problems2-5. Changes in behavior and skin sensitivity (tactile hyper-aesthesia), both with rapid onset, are common clinical signs seen by many practitioners in horses with potential Lyme disease. Neurological signs such as depression, dysphagia, head tilt and encephalitis were reported in chronic cases2,6,7. Most recent reports describe horses with a Borrelia-associated pseudolymphoma8 or Borrelia-associated uveitis9.
The diagnosis of Lyme disease in horses10 can be made based on:
- Horses living in an area where B. burgdorferi infected ticks are endemic
- Horses that have a history of visiting an area with infected ticks
- Ticks that have been found on the horse
- Clinical signs compatible with Lyme disease
- Ruling out other causes of clinical signs that may have similar signs than those associated with Lyme disease, i.e. orthopedic disease, behavioral or training issues
- A high antibody titer to B. burgdorferi
Diagnostic Lyme antibody testing in horse serum has been performed by ELISA followed by a confirmatory Western blot or by immunofluorescence assays (IFA). These assays detected antibodies as early as five to six weeks after infection and resulted in positive antibody titers by ten to twelve weeks in most experimentally infected ponies11. The Equine Lyme Multiplex Assay can identify antibodies to B. burgdorferi by three to five weeks after infection12.
How does the Equine Lyme Multiplex Assay work?
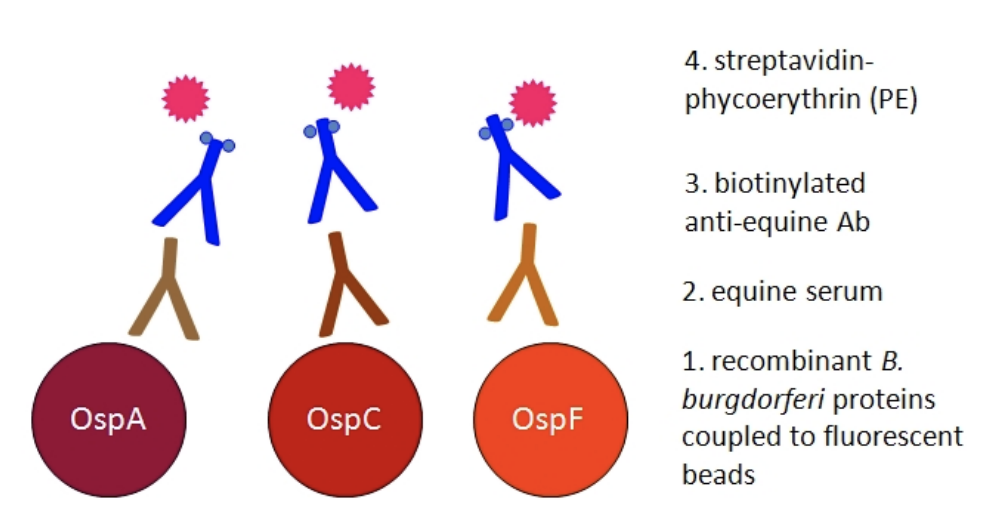
The Equine Lyme Multiplex Assay was developed at the Animal Health Diagnostic Center at Cornell University. It detects antibodies to three antigens of B. burgdorferi in equine serum (Fig. 1). The test is based on fluorescent beads and allows the simultaneous measurement of antibodies to all three B. burgdorferi antigens in a single sample13.
Which B. burgdorferi antigens are used and how is the test interpreted?
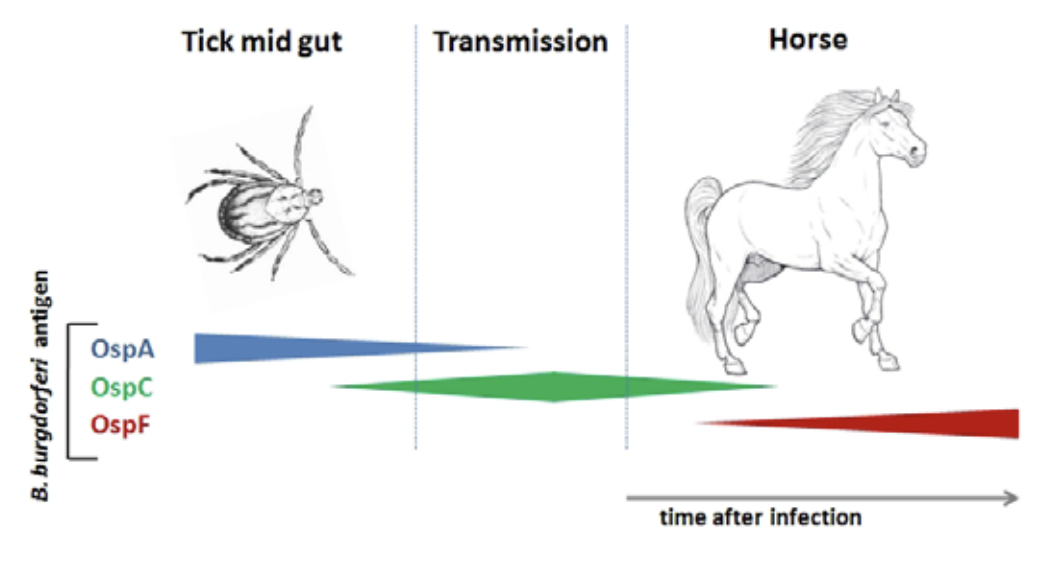
The Equine Lyme Multiplex assay is based on three antigens, called outer surface proteins (Osp), of B. burgdorferi. Various research studies have shown that Osp antigen expression changes on the bacterial surface in response to tick feeding and again after infection of a warm-blooded host, such as dogs, horses, or humans (Fig. 2). In response to infection, horses develop antibodies to these Osp proteins and testing for antibodies to specific Osp antigens can assist in the diagnosis of infection and Lyme disease.
Interpretation of Lyme Multiplex results12,13
The Lyme Multiplex Assay is a fully quantitative test. It results in a numeric antibody value for each of the three B. burgdorferi antigens tested. A brief interpretation on each value is submitted with the test report. In addition, the antibody profile gives an advanced interpretation on the infection and vaccination status of the horse. Antibodies to OspA serve as markers for vaccination and those to OspC and OspF as markers for infection (Fig. 3). In treated horses, quantitative antibody values are valuable indicators to follow-up on treatment success.
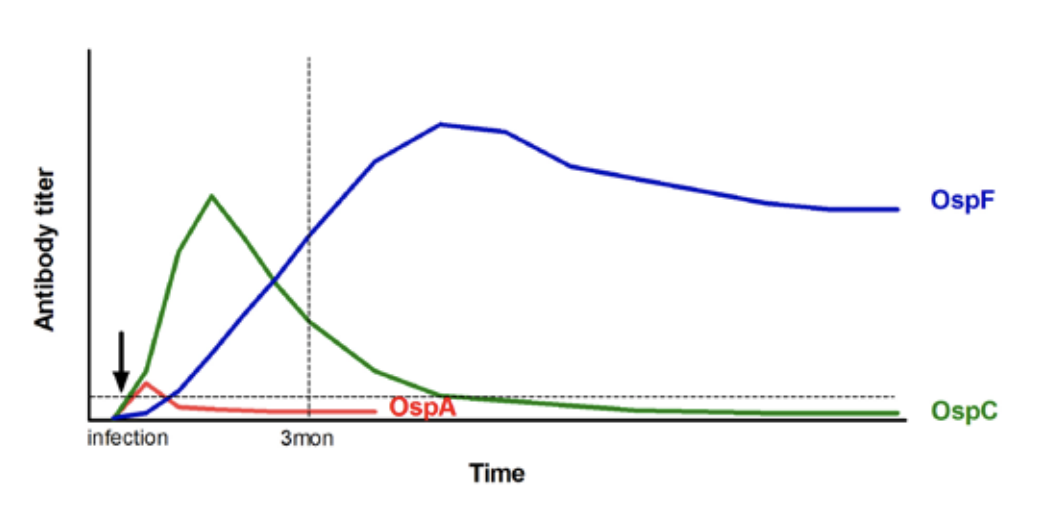
- OspA – positive values for antibodies to OspA are typically observed in vaccinated horses. OspA is expressed while B. burgdorferi persists in the tick mid-gut and also while the bacteria are cultured in-vitro. During infection of mammalian hosts, the bacteria down-regulate OspA. Therefore, antibodies to OspA are generally undetectable after natural infection in most non-vaccinated horses. Low positive, transient antibody values to OspA can sometimes be detected three weeks after infection12.
- OspC – is a valuable indicator of early infection with B. burgdorferi. Antibodies to OspC are detected as early as three weeks after infection. Antibodies to OspC decline after seven to eleven weeks and become undetectable by four to five months after infection12,14.
- OspF – is an indicator of chronic infection. Antibodies to OspF are detectable by five to eight weeks after infection and are maintained at high levels afterwards. Researchers at Cornell observed a very high agreement between antibodies to OspF and C6 as robust markers for infection12,14. Horses with positive antibody values to OspF and negative antibody values to OspC are considered to be infected with B. burgdorferi for at least five months (Fig. 3).
Advantages of the Equine Lyme Multiplex Assay
The Equine Lyme Multiplex Assay is available only at the Animal Health Diagnostic Center at Cornell University. It combines the results obtained by previous ELISA and Western blot testing and exceeds the information obtained by tests that are based on a single antigen of B. burgdorferi such as C6. The Lyme Multiplex Assay provides information whether the horse got infected with B. burgdorferi and when the infection occurred. The test results are fully quantitative and appropriate to follow-up on treatment success or response to vaccination. The advantages of the Lyme Multiplex Assay compared to other Lyme tests are:
-
assay results distinguishing between:
- early and chronic stages of infection; Antibodies to OspC and OspF serve as markers for infection. Antibody values for OspC and OspF can also identify the stage of infection with B. burgdorferi and for how long the horse was infected. In humans, early infection stages have a better prognosis and increased success rates after antibiotic treatment than chronic infection stages. The same trends have been observed in horses. A more rapid decline of antibodies, indicating treatment success, was observed in many horses that were treated in the early infection stage.
- vaccination and infection; The assay provides a separate quantitative value for antibodies to OspA, as a marker for vaccination, and determines the horse’s vaccination status.
- increased specificity and sensitivity; Improved test performance results in earlier detection of antibodies to B. burgdorferi (as early as three weeks after infection) and a higher accuracy of the Lyme multiplex assay compared to conventional Lyme ELISAs.
- quantitative measurement of individual antigens; The Lyme Multiplex Assay has a much broader linear quantification range than ELISAs. Antibody values are precisely measured in a broad concentration range. Quantification of antibodies and evaluation of treatment success can be performed based on declining antibody values. Successful antibiotic treatment decreases the bacterial load. The reduced or missing antigenic stimulus causes the immune system to produce fewer antibodies which then leads to a decrease in serum antibodies against B. burgdorferi. Treatment decisions and follow-up should always be based on a quantitative antibody test. Follow-up by Lyme Multiplex testing can be performed earlier than with conventional ELISAs.
The Lyme Multiplex Assay result provides advanced information beyond any of the currently available Lyme disease testing methods. Lyme Multiplex Assay testing allows a better definition of the horse’s current infection status and assists in determining treatment options and prognosis. The infection status can also be determined most vaccinated horses. Interpretation varies slightly depending on the vaccine used.
Treatment and treatment follow-up using the Equine Lyme Multiplex Assay
For treatment options and recommendations for infected horses with or without clinical signs, we refer to the current literature on Lyme disease in horses: Most commonly used treatments in horses are oxytetracycline (6.6-11.0 mg/kg bwt, i.v., every 24h), doxycycline (10 mg/kg bwt, per os, every 12h)10, or minocycline (4 mg/kg bwt, per os, every 12h). In one experimental study, i.v. oxytetra-cycline treatment seemed to eliminate persistent infection, while three out of four ponies treated per os with doxycycline showed increasing antibody values by three months after the treatment ended15.
In Lyme endemic areas, most practitioners treat horses with clinical signs and a confirmed serological antibody titer to B. burgdorferi with either oral administration of doxycycline or minocycline for thirty to forty-five days, or i.v. oxytetracycline for thirty days, or a combination of oxytetracycline i.v. for a variable period, followed by an oral doxycycline or minocycline treatment for a total treatment time of thirty to sixty days. The duration of treatment is empirical and based on experience. Very few practitioners reported adverse reactions to treatment; the most common side effect was soft manure (B. Wagner and A. Grice: Summary of the Lyme Disease Table Topic – 57th Annual Convention, AAEP, San Antonio, TX, 2011).
In addition to improvement of clinical signs, the evaluation of treatment success is performed based on declining antibody values to B. burgdorferi. The evaluation requires a pre- and post-treatment sample. Successful antibiotic treatment decreases the bacterial load. The lower or missing antigenic stimulus causes the immune system to produce fewer antibodies which leads to a decrease in serum antibody values. The decline of antibodies is slower than the bacterial clearance caused by the antibiotic treatment. After successful treatment, IgG antibodies to B. burgdorferi decline according to their half-life of around twenty-one days. A decrease of a positive antibody value by at least 50% of the original value in the time frames mentioned below is considered an indicator of treatment success. After successful treatment, antibodies will continue to drop without further treatment and will become negative over time if no re-infection occurs. The best timing for follow-up testing depends on the stage of infection. For example:
For chronic infection stages (OspC-/OspF+): Follow-up testing with the Lyme Multiplex Assays can be performed as early as three months after starting treatment. It should not be performed earlier to give the antibodies time to decline. A successful treatment is indicated by a clear drop in OspF antibody values (≥ 50% of the pretreatment value obtained three months earlier). A treatment failure (persistent infection) or reinfection after treatment is indicated by OspF antibody increases, no or minor decreases or alternating antibody values (minor drop followed by increase after re-testing). In case of a reinfection, OspC antibodies will also become positive.
For early infection stages (OspC+/OspF+ or OspC+/OspF-): Treatment follow-up can be performed as early as six weeks after beginning of the treatment. Early in infection, antibodies have been observed to decline more rapidly. This is likely due to a higher amount of IgM antibodies during early infection and a more rapid decline of IgM compared to IgG. A successful treatment is indicated by a clear drop in OspC (and if positive also OspF) antibody values to ≥ 50% of the pretreatment value obtained six weeks earlier. A treatment failure (persistent infection) or reinfection after treatment is indicated by OspC and/or OspF antibody increases, no or minor decreases or alternating antibody values (minor drop followed by increase after re-testing).
Special considerations for vaccinated horses
An approved Lyme vaccine for horses is currently not available. Horses are sometimes vaccinated with one of the three available Lyme vaccines for dogs for attempted protection of horses that are housed in Lyme endemic areas. Efficacy studies of canine vaccines in horses are not yet available, but experimental data suggested that anti-OspA antibodies are protective in horses.
All available vaccines contain OspA antigen as the sole or one of the vaccine components. Antibodies to OspA are identified by the Equine Lyme Multiplex Assay to determine the vaccination status in vaccinated horses. To provide our clients with the best interpretation for each animal, we need information on the vaccine used. This includes the name of the vaccine and the date when the horse was last vaccinated. Please include this information on the submission form when samples of vaccinated horses are submitted for testing.
High OspA antibody values are sometimes observed in non-vaccinated horses (less than 5% of the submitted cases at the AHDC). These cases have been identified previously by Western blotting and are also detected by Equine Lyme Multiplex testing. It is not yet known where these antibodies originate from or what their biological role is in horses with positive OspC and/or OspF antibody values to B. burgdorferi.
Neuroborreliosis in horses
Previous and ongoing research has shown that the Equine Lyme Multiplex Assay can identify antibodies to B. burgdorferi in cerebrospinal fluid (CSF) to confirm neuroborreliosis in horses and to distinguish it from other causes of neurologic disease. The diagnosis is supported by clinical findings, a lymphocytic pleocytosis in the CSF and the identification of antibodies to Borrelia antigens that are locally produced in neural tissues10,16. The latter diagnostic approach requires a comparison of serum and CSF fluid from the same horse. Previous assays were rarely able to detect the overall relatively low Borrelia-specific antibody concentrations in CSF. The Lyme Multiplex Assay can detect much lower amounts of antibodies to Osp antigens and precisely quantify them in CSF. For horses with neurological signs, a serum and CSF sample should be obtained at the same time and submitted together.
How can the Lyme Multiplex Assay be compared to other serological Lyme assays?
Researchers at the Animal Health Diagnostic Center at Cornell University have compared the former ELISA/Western blot procedure and commercial C6- based assays with the Lyme Multiplex Assay12-14. Multiplex Assay OspF and C6 results highly correlate in infected or non-infected dogs12. In horses, comparisons of C6 results and Lyme Multiplex Assay OspF values showed that antibodies to OspF are more robust and the preferred infection markers in horses14. The Equine Lyme Multiplex Assay provides comprehensive information on the horse’s stage of infection and, in vaccinated horses, on the antibody status induced by vaccination.
Sample submission
For detection of antibodies to B. burgdorferi, 2ml of horse serum need to be submitted. Serum should be collected in a red top blood tube. The entire red blood tube or isolated serum should be shipped by overnight shipment on an ice pack to the Animal Health Diagnostic Center at Cornell University. For more information, please see the submission page.
Samples are tested every day (Mon-Fri) and results are available one to two days after the sample arrives at the laboratory. Consultation on Equine Lyme Multiplex Assay interpretation is available by calling the Serology/Immunology laboratory at the Animal Health Diagnostic Center at Cornell University.
References
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochetes. Nature Rev. Microbiol. 10, 87-99.
- Parker JL, White KK. 1992. Lyme borreliosis in cattle and horses: a review of the literature. Cornell Vet. 82, 253-274.
- Egenvall A, Franzén P, Gunnarsson A, Engvall EO, Vågsholm I, Wikström UB, Artursson K. 2001. Crosssectional study of the seroprevalence to Borrelia burgdorferi sensu lato and granulocytic Ehrlichia spp. and demographic, clinical and tick-exposure factors in Swedish horses. Prev. Vet. Med. 49, 191-208.
- Butler CM, Houwers DJ, Jongejan F, van der Kolk JH. 2005. Borrelia burgdorferi infections with special reference to horses. A review. Vet. Q. 27, 146-156.
- Gall Y, Pfister K. 2006. Survey on the subject of equine Lyme borreliosis. Int. J. Med. Microbiol. 40, 274-279.
- James FM, Engiles JB, Beech J. 2010. Meningitis, cranial neuritis, and radiculoneuritis associated with Borrelia burgdorferi infection in a horse. J. Am. Vet. Med. Assoc. 237, 1180-1185.
- Imai DM, Barr BC, Daft B, Bertone JJ, Feng S, Hodzie E, Johnston JM, Olsen KJ, Barthold SW. 2011. Lyme neuroborreliosis in 2 horses. Vet. Pathol. 48, 1151-1157.
- Sears KP, Divers TJ, Neff RT, Miller WH Jr, McDonough SP. 2012. A case of Borrelia-associated pseudolymphoma in a horse. Vet Dermatol. 23, 153- 156.
- Priest HL, Irby NL, Schlafer DH, Divers TJ, Wagner B, Glaser AL, Chang YF, Smith MC. 2012. Diagnosis of Borrelia-associated uveitis in two horses. Vet Ophthalmol., PMID: 22360730.
- Divers TJ, Mair TS, Chang YF. 2009. Lyme disease in horses. In: Mair, T.S., Hutchinson, R.E., eds. Infectious diseases of the horse. Geerings Print Ltd, Ashford, Kent, UK. pp. 286-292.
- Chang YF, Novosol V, McDonough SP, Chang CF, Jacobson RH, Divers T, Quimby FW, Shin S, Lein, DH. 2000. Experimental infection of ponies with Borrelia burgdorferi by exposure to Ixodid ticks. Vet. Pathol. 37, 68-76.
- Wagner B, Freer H, Rollins A, Garcia-Tapia D, Erb HN, et al. 2012. Antibodies to Borrelia burgdorferi OspA, OspC, OspF and C6 antigens as markers for early and late infection in dogs. Clin. Vacc. Immunol., PMID:22336289.
- Wagner B, Freer H, Rollins A, Erb HN, Lu Z, Gröhn Y. 2011. Development of a multiplex assay for detection of antibodies to Borrelia burgdorferi in horses and its validation using Bayesian and conventional statistical methods. Vet. Immunol. Immunopathol., 144: 374-381.
- Wagner B, Goodman LB, Rollins A, Freer HS. 2013. Antibodies to OspC, OspF and C6 antigens as indicators for infection with Borrelia burgdorferi in horses. Equine Vet. J., 45: 533-537.
- Chang YF, Ku YW, Chang CF, Chang CD, McDonough SP, Divers T, Pough M, Torres A. 2005. Antibiotic treatment of experimentally Borrelia burgdorferi-infected ponies. Vet Microbiol. 20, 285- 294.
- Wagner B, Glaser A, Bartol J, Mahar O, Johnson A, Divers T. A new sensitive Lyme multiplex assay to confirm neuroborreliosis in horses – a case report. Proceeding of the AAEP’s 57th Annual Convention, Nov. 18-22, 2011 in San Antonio, Texas.


