DCS Research & Grant Planning Information
- Information outlined below is most often used by faculty performing research in the Department of Clinical Sciences.
- We suggest you start here and work through the resources below. All research projects are unique and we have tried to sequence the materials in the order they are most commonly used, highlighting the areas that tend to cause most questions.
- One or all of these points of contact may help you get started:
- DCS Research Drop-In Sessions, held on the first Wednesday of each month from 1:00-2:00PM via Zoom. These sessions provide an opportunity for an informal discussion of your research idea and help with navigating our research ecosystem. Please contact Maria Hopko for the Zoom link.
- DCS Research Committee members
- Preclinical Services Core (Elizabeth Moore)
- DCS Innovation Laboratory (Mike Byron)
- Clinical Trials Coordinators (Carol Frederick, Cindy Bennett, Andrea King, Adrian Martin)
DCS Innovation Laboratory
The Department of Clinical Sciences operates a fee-based laboratory providing faculty the opportunity to have research conducted on their behalf or to use the lab to perform their own research. This laboratory is ideal for researchers without dedicated lab space, without full-time technicians, or for those who need assistance with projects of a fixed duration.
- The DCS Innovation Laboratory is located in VMC C1118.
- For more information on utilizing the lab, please contact Mike Byron.
Services Available:
- Project Planning
- Supply Purchasing
- Experimentation
- Data Summary/Analysis
DCS Research and Finance Dialogues
The DCS finance team holds a monthly dialogue series focused on financial and research topics.
- For more information, please contact Theresa Lagasse, DCS Finance Manager.
Clinical Trials
If you plan to conduct a clinical trial on client owned animals please contact our Clinical Trials Coordinators (Carol Frederick; Cindy Bennett, Andrea King, Adrian Martin) or visit the Clinical Trials Faculty Resource page for details for setting up a new clinical trial, patient recruitment, and sample collection.
- How to Get Started with Clinical Trials Research pdf. Step by step guide to help develop your project.
- For information on ongoing trials please visit the Clinical Trials homepage.
- CUHA Case Searches for case searches.
- Anivive Clinical Trails web hosting can help get the word out about your clinical trial. Contact Carol Frederick for more information.
CARE
Cornell Center for Animal Resources and Education (CARE) CARE at Cornell University is a service and a resource to the AAALAC accredited Cornell research and teaching community. CARE provides high quality animal care and veterinary services, and advises and educates researchers, staff and students on animal experimentation issues while promoting best practices for the responsible use of animals.
Data Management & Storage
Cornell Data Services (CDS) provides university-wide assistance with a broad range of data needs including writing Data Management Plans (DMP), managing/storing data, guidance on data sharing, and options for archiving and preserving data. Specialists can meet with you for free consultations.
- Data Management Plan (DMP).
- Data Storage
- The CDS Data Storage Finder online interactive tool assists in identifying the best options for data storage.
- The Data Storage Finder spreadsheet may assist you in identifying options.
- Small data sets may be stored in-house on the local CVM K://drive.
- Small to Medium Data Sets may use Cornell Box. https://it.cornell.edu/box
Grants and Funding
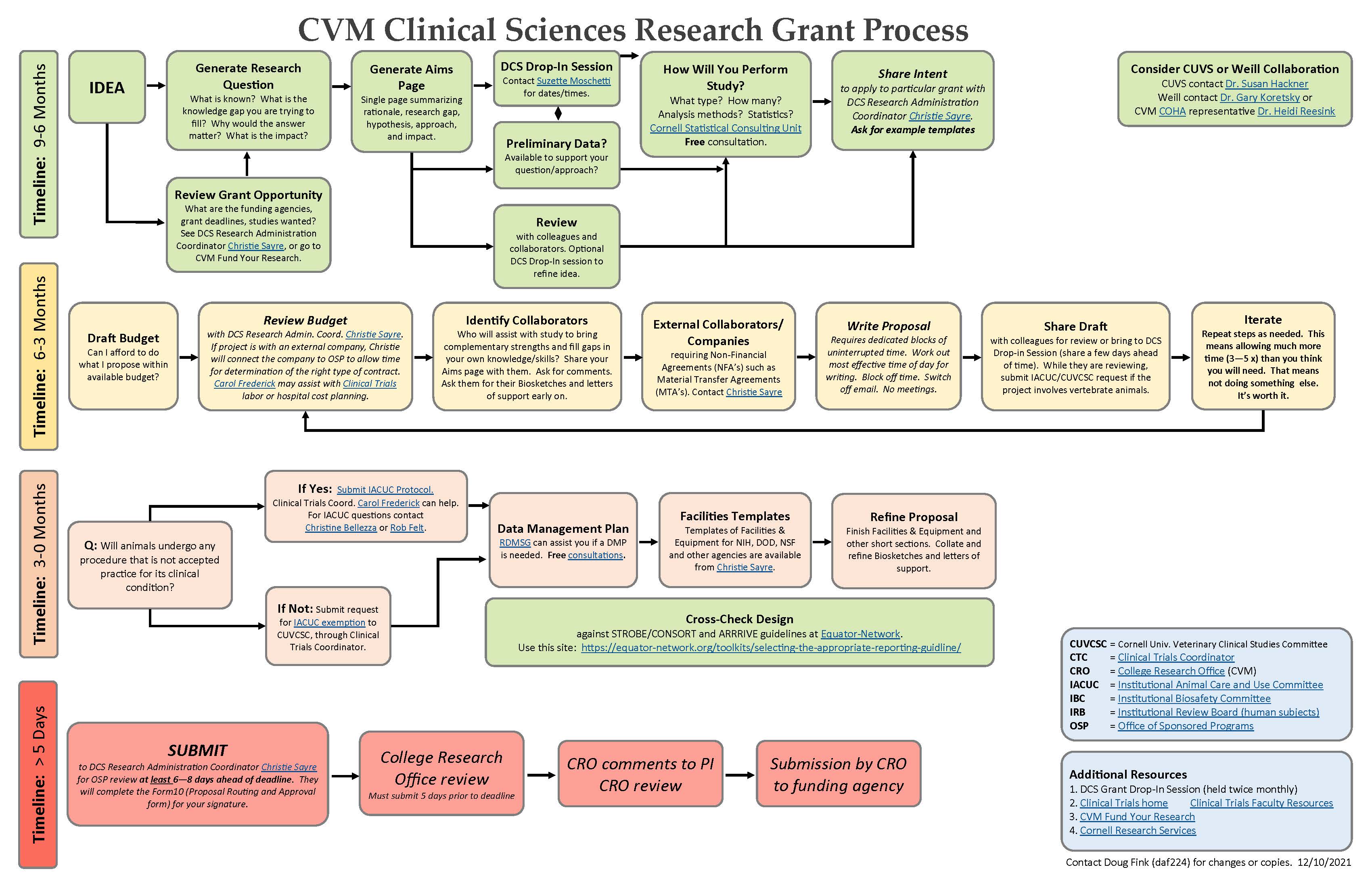
- If you plan to submit a grant to support your project and would like help writing the proposal we recommend that you take a look at this Research Grant Process flow chart. The chart includes a suggested timeline, decision-making process, and contacts for progressing through a research grant application.
- Please also come to one of the DCS Research Drop-In Sessions, held after the DSC faculty meetings. We recommend you do this close to the start of preparing your proposal as we have found faculty can save a lot of energy and time by coming to those sessions early on to refine the ideas and plan the submission.
- To find available opportunities for funding and relevant deadlines contact Christie Sayre, our DCS Grant Coordinator. A full list of possibilities is available at Infoready.
- CVM Fund Your Research also contains much other information to help you plan and budget your proposal.
- Review the ongoing funded work at the CVM Research Awards page to find researchers to collaborate with who may have areas adjacent or overlapping to your own.
- Several faculty members with successful proposals have kindly shared facilities and equipment sections and related materials for NIH, NSF, DOD, Morris Animal Foundation and American Kennel Club proposals with our Department's research coordinator Christie Sayre. Please contact her if one of these templates may be helpful to you.
Clinical and Translational Science Award One Health Alliance (COHA).
Cornell is a member of COHA, the Clinical and Translational Science Award One Health Alliance, which are veterinary schools partnered with medical colleagues to advance our understanding of diseases shared by humans and animals and find solutions & treatments.
- COHA Fellowships Several Cornell research teams are offering fellowship training opportunities for residency-trained veterinarians. Through COHA support, these 2-year research fellowships are designed to engage veterinary clinical specialists in inter-disciplinary and comparative research teams.
- Translational Training Opportunities Cornell faculty, staff and students are encouraged to participate in translational research training opportunities offered through Weill Cornell Medical College and other partner CTSA institutions.
Perpetual Internal DCS Grants
IACUC
IACUC Exemption
If you are using animals as part of your research, you first need to know if you need Institutional Animal Care and Use Committee (IACUC) approval or if your study is exempt. Your study may be exempt from IACUC oversight if:
- You will ONLY use biological materials that would be discarded after standard clinical treatments of the animal for its current condition, or
- If the animal will not undergo ANY clinical procedure that is not standard accepted veterinary practice for its clinical condition.
- If your trial may be exempt, visit our Clinical Trials Faculty Resources page and complete the exemption request.
IACUC Approval
- If you do need an IACUC for your study, then IACUC submission information and gaining access to the system (Cayuse) is available here.
- Advice on completing each section of the IACUC.
- For more help with IACUC’s contact Rob Felt (Senior Administrator) or Christine Belleza (Director of Research Assurance).
Institutional Biosafety Committee (IBC)
Cornell's Institutional Biosafety Committee (IBC) reviews and approves all research and teaching activities involving the use of biohazardous agents.
- All researchers must obtain approval from for activities with recombinant or synthetic nucleic acid molecules (r/sNA) or biohazardous materials.
- Submit a Memorandum of Understanding and Agreement (MUA).
- Research at Biosafety Level -3 requires a separate application.
Statistical Consulting
- Clinically relevant assistance on study design and data analysis is available through the Cornell Statistical Consulting Unit (CSCU).
- Both CSCU's director Lynn Johnson (lms86@cornell.edu) and consultant Stephen Parry (stephen.parry@cornell.edu) work with many of our clinicians and may be contacted directly.
- Ideally, contact the Cornell Statistical Consulting Unit before you start your study.
- JMP Student Edition is available FREE to all students, educators, and instructional staff at schools, colleges, and universities. If greater statistical power is required or datasets are extremely large, you may contact CSCU consultants for assistance.
CSCU CONSULTING (free):
- Researchers from units that financially support CSCU (includes all CVM) can receive unlimited free statistical consulting.
- For all other Cornell affiliated clients the rate is $165/hr.
- Consultants meet with faculty and staff and offer advice on anything related to the design of their study/experiment and the statistical analysis of their data (e.g., sample size calculations, selecting an appropriate statistical analysis, using statistical software packages etc.).
- Consultants do not perform any of the analysis for clients; consultants share expertise and advice to enable researchers to perform the analysis on their own.
- For short questions, you can visit us during their daily drop-in times. For longer questions, please schedule an appointment.
CSCU CONTRACT ($165/hr):
- A contract project can be initiated by a Cornell faculty or staff member who would like to have their statistical analysis completed by a CSCU statistician.
- Needs and availability for this service are discussed on a project-by-project basis.
- If CSCU performs computation or analysis for you, then it is a contract and you will be billed at the contract rate.
- The rate for contract work provided by a CSCU staff statistician is $165/hr for all projects with Cornell affiliated clients (this includes CVM)
Additional Resources Available
Environmental Health & Safety
Environmental Health and Safety - Assists the campus community in promoting health, safety and environmental stewardship.
- The 'Ask EHS' on-line question and answer system is the best way for the Cornell community to ask EHS questions of the expert staff in all aspects of environment, health and safety.
- Ask EHS Inquiries submitted will be answered by EHS field experts.
Laboratory Safety
Lab Safety Training
- For questions not answered below, or assistance with lab-specific issues, please contact Doug Fink (607-280-9361), Clinical Sciences Material & Safety Coordinator.
- All new laboratory personnel must complete the DCS Laboratory Safety Training Checklist. Doug Fink will review the checklist with PI's and technicians. PI's or technician's are expected to review the form with all subsequent lab personnel, including post-docs, residents, DVM and undergraduate students. Signed copies of the Checklist should be kept on file with the PI or lab.
Biomedical Waste Disposal
- 'CVM Medical Waste Disposal Guide' pdf provides information on disposal methods for different laboratory waste streams.
- All medical, bio, and carcass waste must be submitted to the CVM Waste Management Facility (WMF).
- Biowaste (including sharps and chemotheraputic waste) costs the lab $0.64/pound (2025).
- All biowaste must be transported in red carts - no exceptions. Carts shall not pass by the cafe - go around or use the 1st floor to cross the Atrium.
- All waste submitted to CVM WMF must be accompanied by a Medical Waste Tracking Tag.
- Instructions for printing a Medical Waste Tracking Tag.
- Microsoft Internet Explorer browser is recommended for printing tags. Must be able to print barcode.
- If barcode doesn't print after following instructions, contact VMIT or Doug Fink to request VMIT adjust the computer settings.
Chemical Handling & Waste Disposal
- Chemical Abbreviation Key provides a list of commonly used chemical abbreviations to use on bottles in lab.
- Chemical Segregation Chart provides recommendations for chemical segregation and storage.
- Chemwatch Cornell licensed database for chemical Safety Data Sheets. Also provides for printing bottle labels.
- Hazardous chemical waste is collected and disposed by EHS free of charge to the PI.
- ALL containers must be labeled with 1) "Hazardous Waste", 2) full name of the chemical(s), and 3) the date container first filled.
- Use green EHS Haz Waste Labels on every container to meet the above conditions and to facilitate pickup by EHS.
- All waste must be placed in DOT Haz Waste shipping boxes prior to pickup. Empty boxes may be found in the CVM Recycling Room.
- 'Hazardous Waste Removal Form' must be completed to schedule an EHS Haz Waste Pickup. Pickups made on Wednesday and Friday.
- Unknown Chemical Characterization Form provides for a way to dispose of unlabeled chemicals. Completion of both a Haz Waste Tag and Unknowns form.
- 'Down the Drain' link may be used as a guide to dispose some chemicals down the waste water drain. Please exercise caution and prudence.
Controlled Substances
- Controlled substances purchased for animals with clinical case numbers may be made through the CUHA Pharmacy.
- All controlled substances purchased for Research or Teaching purposes must be made through Doug Fink on the departmental license.
- In order to purchase controlled drugs on the department licenses, each PI must complete three steps:
- Complete online training. Doug Fink can assign this training upon request.
- Complete the DEA required 'Personnel Screening Questionnaire' and return to Doug Fink.
- Complete the Controlled Substance Purchase Request form for each purchase made.
- See the Cornell EHS Controlled Substances Policy for more information.
NYS Hypodermic Needle & Syringe Policy (N&S)
- The Department of Clinical Sciences maintains a NYS Certificate of Need (CON) for research outside CUHA.
- The CON includes all hypodermic needles & syringes only. Does not include irrigation syringes.
- Main points each PI/Lab must follow include:
- Essential persons shall sign a form indicating they will follow NYS law. Supervisor, Custodian, User.
- All hypodermic N&S must be stored in a locked location. Drawer, cabinet, locker.
- Inventory records must be maintained. Quantities received and issued must be recorded in and out.
- Reconciliation Inventory must be completed each June 30. Update inventory records.
- See the Cornell EHS Control of Hypodermic Syringes and Needles Guidance Document for more information.
- Contact Doug Fink for forms and to be added to the DCS Certificate of Need.
Liquid Nitrogen (LN2)
- LN2 is available at the following locations: VMC C3100 Lab Hallway (West), VRT T4009.
- The Use Log must be completed for each withdrawal, including: Date, Name, PI, Account# to charge, Amount Withdrawn.
- If the VMC tank is empty, or there are any issues with the tanks, please contact Doug Fink (607-280-9361).
Recycling Policy
- CVM Recycling Policy
- Only clean, uncontaminated, paper products may be placed in laboratory recycling containers.
- Pipet boxes may be submitted to the manufacturer for recycling (see CVM Recycling Policy). Pipet tips must be disposed as sharps.
Forms & Miscellaneous
- Cornell Laboratory Safety Manual & Chemical Hygiene Plan dd .
- Cornell Labs Top 10 List outlines
- Research Laboratory Inspection Checklist provides
- Eyewash Testing Log tracks
- NYS Needle & Syringe Policy
- CU Guidance Document
- MOU's for Supervisor, Custodian, and Users
- Inventory Log Blanks
- Laundering Laboratory Coats - DFA resource outlining approved vendor options for laundering laboratory coats.